[English] 日本語
 Yorodumi
Yorodumi- EMDB-44593: Cryo-EM structure of human CHT1 in the HC-3 bound outward-facing state -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human CHT1 in the HC-3 bound outward-facing state | ||||||||||||
 Map data Map data | structure of human CHT1 in the HC-3 bound outward-facing state | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Choline transporter / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcholine:sodium symporter activity / Defective SLC5A7 causes distal hereditary motor neuronopathy 7A (HMN7A) / Defective SLC5A7 causes distal hereditary motor neuronopathy 7A (HMN7A) / acetylcholine biosynthetic process / : / choline transmembrane transporter activity / Acetylcholine Neurotransmitter Release Cycle / choline transport / neuromuscular synaptic transmission / choline binding ...choline:sodium symporter activity / Defective SLC5A7 causes distal hereditary motor neuronopathy 7A (HMN7A) / Defective SLC5A7 causes distal hereditary motor neuronopathy 7A (HMN7A) / acetylcholine biosynthetic process / : / choline transmembrane transporter activity / Acetylcholine Neurotransmitter Release Cycle / choline transport / neuromuscular synaptic transmission / choline binding / synaptic transmission, cholinergic / neurotransmitter transport / neuromuscular junction / transmembrane transport / synaptic vesicle membrane / presynaptic membrane / early endosome membrane / perikaryon / in utero embryonic development / axon / synapse / dendrite / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.67 Å | ||||||||||||
 Authors Authors | Xue J / Jiang Y | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2024 Journal: Cell Discov / Year: 2024Title: Structural mechanisms of human sodium-coupled high-affinity choline transporter CHT1. Authors: Jing Xue / Hongwen Chen / Yong Wang / Youxing Jiang /   Abstract: Mammalian sodium-coupled high-affinity choline transporter CHT1 uptakes choline in cholinergic neurons for acetylcholine synthesis and plays a critical role in cholinergic neurotransmission. Here, we ...Mammalian sodium-coupled high-affinity choline transporter CHT1 uptakes choline in cholinergic neurons for acetylcholine synthesis and plays a critical role in cholinergic neurotransmission. Here, we present the high-resolution cryo-EM structures of human CHT1 in apo, substrate- and ion-bound, hemicholinium-3-inhibited, and ML352-inhibited states. These structures represent three distinct conformational states, elucidating the structural basis of the CHT1-mediated choline uptake mechanism. Three ion-binding sites, two for Na and one for Cl, are unambiguously defined in the structures, demonstrating that both ions are indispensable cofactors for high-affinity choline-binding and are likely transported together with the substrate in a 2:1:1 stoichiometry. The two inhibitor-bound CHT1 structures reveal two distinct inhibitory mechanisms and provide a potential structural platform for designing therapeutic drugs to manipulate cholinergic neuron activity. Combined with the functional analysis, this study provides a comprehensive view of the structural mechanisms underlying substrate specificity, substrate/ion co-transport, and drug inhibition of a physiologically important symporter. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44593.map.gz emd_44593.map.gz | 49.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44593-v30.xml emd-44593-v30.xml emd-44593.xml emd-44593.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44593_fsc.xml emd_44593_fsc.xml | 7.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_44593.png emd_44593.png | 68.8 KB | ||
| Filedesc metadata |  emd-44593.cif.gz emd-44593.cif.gz | 6.2 KB | ||
| Others |  emd_44593_half_map_1.map.gz emd_44593_half_map_1.map.gz emd_44593_half_map_2.map.gz emd_44593_half_map_2.map.gz | 49 MB 49 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44593 http://ftp.pdbj.org/pub/emdb/structures/EMD-44593 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44593 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44593 | HTTPS FTP |
-Validation report
| Summary document |  emd_44593_validation.pdf.gz emd_44593_validation.pdf.gz | 926.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44593_full_validation.pdf.gz emd_44593_full_validation.pdf.gz | 926.4 KB | Display | |
| Data in XML |  emd_44593_validation.xml.gz emd_44593_validation.xml.gz | 15.7 KB | Display | |
| Data in CIF |  emd_44593_validation.cif.gz emd_44593_validation.cif.gz | 19.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44593 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44593 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44593 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44593 | HTTPS FTP |
-Related structure data
| Related structure data |  9bimMC  9bfiC  9bfjC  9bfkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44593.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44593.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | structure of human CHT1 in the HC-3 bound outward-facing state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map A
| File | emd_44593_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_44593_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human CHT1 in the HC-3 bound state
| Entire | Name: human CHT1 in the HC-3 bound state |
|---|---|
| Components |
|
-Supramolecule #1: human CHT1 in the HC-3 bound state
| Supramolecule | Name: human CHT1 in the HC-3 bound state / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: High affinity choline transporter 1
| Macromolecule | Name: High affinity choline transporter 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.526625 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAFHVEGLIA IIVFYLLILL VGIWAAWRTK NSGSAEERSE AIIVGGRDIG LLVGGFTMTA TWVGGGYING TAEAVYVPGY GLAWAQAPI GYSLSLILGG LFFAKPMRSK GYVTMLDPFQ QIYGKRMGGL LFIPALMGEM FWAAAIFSAL GATISVIIDV D MHISVIIS ...String: MAFHVEGLIA IIVFYLLILL VGIWAAWRTK NSGSAEERSE AIIVGGRDIG LLVGGFTMTA TWVGGGYING TAEAVYVPGY GLAWAQAPI GYSLSLILGG LFFAKPMRSK GYVTMLDPFQ QIYGKRMGGL LFIPALMGEM FWAAAIFSAL GATISVIIDV D MHISVIIS ALIATLYTLV GGLYSVAYTD VVQLFCIFVG LWISVPFALS HPAVADIGFT AVHAKYQKPW LGTVDSSEVY SW LDSFLLL MLGGIPWQAY FQRVLSSSSA TYAQVLSFLA AFGCLVMAIP AILIGAIGAS TDWNQTAYGL PDPKTTEEAD MIL PIVLQY LCPVYISFFG LGAVSAAVMS SADSSILSAS SMFARNIYQL SFRQNASDKE IVWVMRITVF VFGASATAMA LLTK TVYGL WYLSSDLVYI VIFPQLLCVL FVKGTNTYGA VAGYVSGLFL RITGGEPYLY LQPLIFYPGY YPDDNGIYNQ KFPFK TLAM VTSFLTNICI SYLAKYLFES GTLPPKLDVF DAVVARHSEE NMDKTILVKN ENIKLDELAL VKPRQSMTLS STFTNK EAF LDVDSSPEGS GTEDNLQAAA GGSWSHPQFE KGGGSGGGSG GSAWSHPQFE K UniProtKB: High affinity choline transporter 1 |
-Macromolecule #2: (2S,2'S)-2,2'-biphenyl-4,4'-diylbis(2-hydroxy-4,4-dimethylmorphol...
| Macromolecule | Name: (2S,2'S)-2,2'-biphenyl-4,4'-diylbis(2-hydroxy-4,4-dimethylmorpholin-4-ium) type: ligand / ID: 2 / Number of copies: 1 / Formula: HC6 |
|---|---|
| Molecular weight | Theoretical: 414.538 Da |
| Chemical component information | 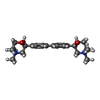 ChemComp-HC6: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





































