[English] 日本語
 Yorodumi
Yorodumi- EMDB-44458: 80S ribosome with angiogenin, in vitro assembled complex without ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 80S ribosome with angiogenin, in vitro assembled complex without substrate tRNA | |||||||||
 Map data Map data | 80S ribosome with angiogenin, in vitro assembled complex without substrate tRNA | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Angiogenin / RNase / RIBOSOME | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
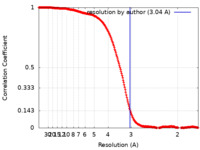
| Method | single particle reconstruction / cryo EM / Resolution: 3.04 Å | |||||||||
 Authors Authors | Loveland AB / Korostelev AA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structural mechanism of angiogenin activation by the ribosome. Authors: Anna B Loveland / Cha San Koh / Robin Ganesan / Allan Jacobson / Andrei A Korostelev /  Abstract: Angiogenin, an RNase-A-family protein, promotes angiogenesis and has been implicated in cancer, neurodegenerative diseases and epigenetic inheritance. After activation during cellular stress, ...Angiogenin, an RNase-A-family protein, promotes angiogenesis and has been implicated in cancer, neurodegenerative diseases and epigenetic inheritance. After activation during cellular stress, angiogenin cleaves tRNAs at the anticodon loop, resulting in translation repression. However, the catalytic activity of isolated angiogenin is very low, and the mechanisms of the enzyme activation and tRNA specificity have remained a puzzle. Here we identify these mechanisms using biochemical assays and cryogenic electron microscopy (cryo-EM). Our study reveals that the cytosolic ribosome is the activator of angiogenin. A cryo-EM structure features angiogenin bound in the A site of the 80S ribosome. The C-terminal tail of angiogenin is rearranged by interactions with the ribosome to activate the RNase catalytic centre, making the enzyme several orders of magnitude more efficient in tRNA cleavage. Additional 80S-angiogenin structures capture how tRNA substrate is directed by the ribosome into angiogenin's active site, demonstrating that the ribosome acts as the specificity factor. Our findings therefore suggest that angiogenin is activated by ribosomes with a vacant A site, the abundance of which increases during cellular stress. These results may facilitate the development of therapeutics to treat cancer and neurodegenerative diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44458.map.gz emd_44458.map.gz | 794.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44458-v30.xml emd-44458-v30.xml emd-44458.xml emd-44458.xml | 15.6 KB 15.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44458_fsc.xml emd_44458_fsc.xml | 20.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_44458.png emd_44458.png | 102.5 KB | ||
| Filedesc metadata |  emd-44458.cif.gz emd-44458.cif.gz | 4.3 KB | ||
| Others |  emd_44458_half_map_1.map.gz emd_44458_half_map_1.map.gz emd_44458_half_map_2.map.gz emd_44458_half_map_2.map.gz | 144.4 MB 144.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44458 http://ftp.pdbj.org/pub/emdb/structures/EMD-44458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44458 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_44458.map.gz / Format: CCP4 / Size: 857.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44458.map.gz / Format: CCP4 / Size: 857.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 80S ribosome with angiogenin, in vitro assembled complex without substrate tRNA | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.87 Å | ||||||||||||||||||||||||||||||||||||
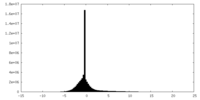
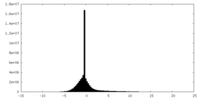
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: 80S ribosome with angiogenin, in vitro assembled complex...
| File | emd_44458_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 80S ribosome with angiogenin, in vitro assembled complex without substrate tRNA, half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
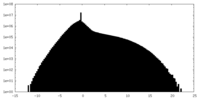
| Density Histograms |
-Half map: 80S ribosome with angiogenin, in vitro assembled complex...
| File | emd_44458_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 80S ribosome with angiogenin, in vitro assembled complex without substrate tRNA, half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
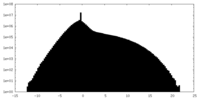
| Density Histograms |
- Sample components
Sample components
-Entire : 80S ribosome with angiogenin assembled in vitro without substrate tRNA
| Entire | Name: 80S ribosome with angiogenin assembled in vitro without substrate tRNA |
|---|---|
| Components |
|
-Supramolecule #1: 80S ribosome with angiogenin assembled in vitro without substrate tRNA
| Supramolecule | Name: 80S ribosome with angiogenin assembled in vitro without substrate tRNA type: complex / ID: 1 / Parent: 0 |
|---|---|
| Molecular weight | Theoretical: 4.5 MDa |
-Supramolecule #2: 80S ribosome
| Supramolecule | Name: 80S ribosome / type: complex / ID: 2 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: angiogenin
| Supramolecule | Name: angiogenin / type: complex / ID: 3 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #4: tRNA-fMet
| Supramolecule | Name: tRNA-fMet / type: complex / ID: 4 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #5: mRNA
| Supramolecule | Name: mRNA / type: complex / ID: 5 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Synthetically produced: Yes Homo sapiens (human) / Synthetically produced: Yes |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)