[English] 日本語
 Yorodumi
Yorodumi- EMDB-4427: The structure of a di-ribosome (disome) as a unit for RQC and NGD... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4427 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of a di-ribosome (disome) as a unit for RQC and NGD quality control pathways recognition. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ribosome / disome / di-ribosome / stalling / TRANSLATION | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmaturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, LSU-rRNA,5S) / regulation of amino acid metabolic process / negative regulation of glucose mediated signaling pathway / translational readthrough / positive regulation of translational fidelity / : / RMTs methylate histone arginines / Protein methylation / mTORC1-mediated signalling / Protein hydroxylation ...maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, LSU-rRNA,5S) / regulation of amino acid metabolic process / negative regulation of glucose mediated signaling pathway / translational readthrough / positive regulation of translational fidelity / : / RMTs methylate histone arginines / Protein methylation / mTORC1-mediated signalling / Protein hydroxylation / ribosome-associated ubiquitin-dependent protein catabolic process / pre-mRNA 5'-splice site binding / GDP-dissociation inhibitor activity / cytosolic large ribosomal subunit assembly / positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / nonfunctional rRNA decay / Formation of the ternary complex, and subsequently, the 43S complex / Translation initiation complex formation / response to cycloheximide / Ribosomal scanning and start codon recognition / cleavage in ITS2 between 5.8S rRNA and LSU-rRNA of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / preribosome, small subunit precursor / Major pathway of rRNA processing in the nucleolus and cytosol / mRNA destabilization / SRP-dependent cotranslational protein targeting to membrane / GTP hydrolysis and joining of the 60S ribosomal subunit / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / positive regulation of protein kinase activity / Formation of a pool of free 40S subunits / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / L13a-mediated translational silencing of Ceruloplasmin expression / negative regulation of translational frameshifting / ribosomal large subunit export from nucleus / translational elongation / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / G-protein alpha-subunit binding / Ub-specific processing proteases / 90S preribosome / ribosomal subunit export from nucleus / translational termination / regulation of translational fidelity / protein-RNA complex assembly / maturation of LSU-rRNA / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / ribosomal small subunit export from nucleus / translation regulator activity / DNA-(apurinic or apyrimidinic site) endonuclease activity / rescue of stalled cytosolic ribosome / cellular response to amino acid starvation / protein kinase C binding / ribosome assembly / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / macroautophagy / maturation of SSU-rRNA / translational initiation / small-subunit processome / maintenance of translational fidelity / modification-dependent protein catabolic process / protein tag activity / cytoplasmic stress granule / rRNA processing / ribosome biogenesis / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / negative regulation of translation / rRNA binding / structural constituent of ribosome / protein ubiquitination / ribosome / translation / G protein-coupled receptor signaling pathway / negative regulation of gene expression / response to antibiotic / mRNA binding / ubiquitin protein ligase binding / nucleolus / mitochondrion / RNA binding / zinc ion binding / nucleoplasm / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
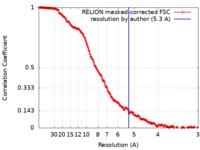
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | ||||||||||||
 Authors Authors | Tesina P / Cheng J | ||||||||||||
| Funding support |  Germany, Germany,  Japan, 3 items Japan, 3 items
| ||||||||||||
 Citation Citation |  Journal: EMBO J / Year: 2019 Journal: EMBO J / Year: 2019Title: Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. Authors: Ken Ikeuchi / Petr Tesina / Yoshitaka Matsuo / Takato Sugiyama / Jingdong Cheng / Yasushi Saeki / Keiji Tanaka / Thomas Becker / Roland Beckmann / Toshifumi Inada /   Abstract: Ribosome stalling triggers quality control pathways targeting the mRNA (NGD: no-go decay) and the nascent polypeptide (RQC: ribosome-associated quality control). RQC requires Hel2-dependent uS10 ...Ribosome stalling triggers quality control pathways targeting the mRNA (NGD: no-go decay) and the nascent polypeptide (RQC: ribosome-associated quality control). RQC requires Hel2-dependent uS10 ubiquitination and the RQT complex in yeast. Here, we report that Hel2-dependent uS10 ubiquitination and Slh1/Rqt2 are crucial for RQC and NGD induction within a di-ribosome (disome) unit, which consists of the leading stalled ribosome and the following colliding ribosome. Hel2 preferentially ubiquitinated a disome over a monosome on a quality control inducing reporter mRNA in an translation reaction. Cryo-EM analysis of the disome unit revealed a distinct structural arrangement suitable for recognition and modification by Hel2. The absence of the RQT complex or uS10 ubiquitination resulted in the elimination of NGD within the disome unit. Instead, we observed Hel2-mediated cleavages upstream of the disome, governed by initial Not4-mediated monoubiquitination of eS7 and followed by Hel2-mediated K63-linked polyubiquitination. We propose that Hel2-mediated ribosome ubiquitination is required both for canonical NGD (NGD) and RQC coupled to the disome and that RQC-uncoupled NGD outside the disome (NGD) can occur in a Not4-dependent manner. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4427.map.gz emd_4427.map.gz | 260.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4427-v30.xml emd-4427-v30.xml emd-4427.xml emd-4427.xml | 100.2 KB 100.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4427_fsc.xml emd_4427_fsc.xml | 17.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_4427.png emd_4427.png | 52.5 KB | ||
| Filedesc metadata |  emd-4427.cif.gz emd-4427.cif.gz | 19.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4427 http://ftp.pdbj.org/pub/emdb/structures/EMD-4427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4427 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4427 | HTTPS FTP |
-Related structure data
| Related structure data |  6i7oMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4427.map.gz / Format: CCP4 / Size: 494.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4427.map.gz / Format: CCP4 / Size: 494.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4996 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Cryo-EM structure of a native stalled di-ribosome (disome) comple...
+Supramolecule #1: Cryo-EM structure of a native stalled di-ribosome (disome) comple...
+Macromolecule #1: 25S ribosomal RNA
+Macromolecule #2: 5S ribosomal RNA
+Macromolecule #3: 5.8S ribosomal RNA
+Macromolecule #18: 18S ribosomal RNA
+Macromolecule #80: P-site tRNA
+Macromolecule #81: E-site tRNA
+Macromolecule #82: A/P hybrid tRNA
+Macromolecule #83: P/E hybrid tRNA
+Macromolecule #84: mRNA
+Macromolecule #4: 60S ribosomal protein L2-A
+Macromolecule #5: 60S ribosomal protein L3
+Macromolecule #6: 60S ribosomal protein L4-A
+Macromolecule #7: 60S ribosomal protein L5
+Macromolecule #8: 60S ribosomal protein L6-A
+Macromolecule #9: 60S ribosomal protein L7-A
+Macromolecule #10: 60S ribosomal protein L8-A
+Macromolecule #11: 60S ribosomal protein L9-A
+Macromolecule #12: 60S ribosomal protein L10
+Macromolecule #13: 60S ribosomal protein L11-B
+Macromolecule #14: 60S ribosomal protein L13-A
+Macromolecule #15: 60S ribosomal protein L14-A
+Macromolecule #16: 60S ribosomal protein L15-A
+Macromolecule #17: 60S ribosomal protein L16-A
+Macromolecule #19: 40S ribosomal protein S0-A
+Macromolecule #20: 40S ribosomal protein S1-A
+Macromolecule #21: 40S ribosomal protein S2
+Macromolecule #22: 40S ribosomal protein S3
+Macromolecule #23: 40S ribosomal protein S4-A
+Macromolecule #24: 40S ribosomal protein S5
+Macromolecule #25: 40S ribosomal protein S6-A
+Macromolecule #26: 40S ribosomal protein S7-A
+Macromolecule #27: 40S ribosomal protein S8-A
+Macromolecule #28: 40S ribosomal protein S9-A
+Macromolecule #29: 40S ribosomal protein S10-A
+Macromolecule #30: 40S ribosomal protein S11-A
+Macromolecule #31: 40S ribosomal protein S12
+Macromolecule #32: 40S ribosomal protein S13
+Macromolecule #33: 40S ribosomal protein S14-B
+Macromolecule #34: 40S ribosomal protein S15
+Macromolecule #35: 40S ribosomal protein S16-A
+Macromolecule #36: 40S ribosomal protein S17-A
+Macromolecule #37: 40S ribosomal protein S18-A
+Macromolecule #38: 40S ribosomal protein S19-A
+Macromolecule #39: 40S ribosomal protein S20
+Macromolecule #40: 40S ribosomal protein S21-A
+Macromolecule #41: 40S ribosomal protein S22-A
+Macromolecule #42: 40S ribosomal protein S23-A
+Macromolecule #43: 40S ribosomal protein S24-A
+Macromolecule #44: 40S ribosomal protein S25-A
+Macromolecule #45: 40S ribosomal protein S26-B
+Macromolecule #46: 40S ribosomal protein S27-A
+Macromolecule #47: 40S ribosomal protein S28-A
+Macromolecule #48: 40S ribosomal protein S29-A
+Macromolecule #49: 40S ribosomal protein S30-A
+Macromolecule #50: Ubiquitin-40S ribosomal protein S31
+Macromolecule #51: Guanine nucleotide-binding protein subunit beta-like protein
+Macromolecule #52: 60S ribosomal protein L17-A
+Macromolecule #53: 60S ribosomal protein L18-A
+Macromolecule #54: 60S ribosomal protein L19-A
+Macromolecule #55: 60S ribosomal protein L20-A
+Macromolecule #56: 60S ribosomal protein L21-A
+Macromolecule #57: 60S ribosomal protein L22-A
+Macromolecule #58: 60S ribosomal protein L23-A
+Macromolecule #59: 60S ribosomal protein L24-A
+Macromolecule #60: 60S ribosomal protein L25
+Macromolecule #61: 60S ribosomal protein L26-A
+Macromolecule #62: 60S ribosomal protein L27-A
+Macromolecule #63: 60S ribosomal protein L28
+Macromolecule #64: 60S ribosomal protein L29
+Macromolecule #65: 60S ribosomal protein L30
+Macromolecule #66: 60S ribosomal protein L31-A
+Macromolecule #67: 60S ribosomal protein L32
+Macromolecule #68: 60S ribosomal protein L33-A
+Macromolecule #69: 60S ribosomal protein L34-A
+Macromolecule #70: 60S ribosomal protein L35-A
+Macromolecule #71: 60S ribosomal protein L36-A
+Macromolecule #72: 60S ribosomal protein L37-A
+Macromolecule #73: 60S ribosomal protein L38
+Macromolecule #74: 60S ribosomal protein L39
+Macromolecule #75: Ubiquitin-60S ribosomal protein L40
+Macromolecule #76: 60S ribosomal protein L41-B
+Macromolecule #77: 60S ribosomal protein L42-A
+Macromolecule #78: 60S ribosomal protein L43-A
+Macromolecule #79: 60S acidic ribosomal protein P0
+Macromolecule #85: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 2.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)