[English] 日本語
 Yorodumi
Yorodumi- EMDB-43855: Ternary complex of human DNA polymerase theta polymerase domain w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ternary complex of human DNA polymerase theta polymerase domain with a cognate C:G base pair | |||||||||
 Map data Map data | Ternary complex of human DNA polymerase theta polymerase domain with a cognate C:G base pair | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA translesion synthesis / theta-mediated end joining / A-family DNA polymerase / DNA BINDING PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationdouble-strand break repair via alternative nonhomologous end joining / HDR through MMEJ (alt-NHEJ) / single-stranded DNA helicase activity / replication fork processing / mitochondrial nucleoid / site of DNA damage / 5'-deoxyribose-5-phosphate lyase activity / error-prone translesion synthesis / negative regulation of double-strand break repair via homologous recombination / somatic hypermutation of immunoglobulin genes ...double-strand break repair via alternative nonhomologous end joining / HDR through MMEJ (alt-NHEJ) / single-stranded DNA helicase activity / replication fork processing / mitochondrial nucleoid / site of DNA damage / 5'-deoxyribose-5-phosphate lyase activity / error-prone translesion synthesis / negative regulation of double-strand break repair via homologous recombination / somatic hypermutation of immunoglobulin genes / DNA helicase activity / base-excision repair / protein homooligomerization / RNA-directed DNA polymerase / RNA-directed DNA polymerase activity / double-strand break repair / site of double-strand break / DNA helicase / DNA-directed DNA polymerase / damaged DNA binding / DNA-directed DNA polymerase activity / DNA repair / DNA damage response / chromatin binding / magnesium ion binding / Golgi apparatus / ATP hydrolysis activity / nucleoplasm / ATP binding / identical protein binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.11 Å | |||||||||
 Authors Authors | Li C / Gao Y | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structural basis of error-prone DNA synthesis by DNA polymerase θ. Authors: Chuxuan Li / Leora M Maksoud / Yang Gao /  Abstract: DNA polymerase θ (Pol θ) is an A-family DNA polymerase specialized in DNA double-strand breaks repair and translesion synthesis. Distinct from its high-fidelity homologs in DNA replication, Pol θ ...DNA polymerase θ (Pol θ) is an A-family DNA polymerase specialized in DNA double-strand breaks repair and translesion synthesis. Distinct from its high-fidelity homologs in DNA replication, Pol θ catalyzes template-dependent DNA synthesis with an inherent propensity for error incorporation. However, the structural basis of Pol θ's low-fidelity DNA synthesis is not clear. Here, we present cryo-electron microscopy structures detailing the polymerase domain of human Pol θ in complex with a cognate C:G base pair (bp), a mismatched T:G bp, or a mismatched T:T bp. Our structures illustrate that Pol θ snugly accommodates the mismatched nascent base pairs within its active site with the finger domain well-closed, consistent with our in-solution fluorescence measurement but in contrast to its high-fidelity homologs. In addition, structural examination and mutagenesis study show that unique residues surrounding the active site contribute to the stabilization of the mismatched nascent base pair. Furthermore, Pol θ can efficiently extend from the misincorporated T:G or T:T mismatches, yet with a preference for template or primer looping-out, resulting in insertions and deletions. Collectively, our results elucidate how an A-family polymerase is adapted for error-prone DNA synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43855.map.gz emd_43855.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43855-v30.xml emd-43855-v30.xml emd-43855.xml emd-43855.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43855.png emd_43855.png | 94.4 KB | ||
| Filedesc metadata |  emd-43855.cif.gz emd-43855.cif.gz | 6.7 KB | ||
| Others |  emd_43855_half_map_1.map.gz emd_43855_half_map_1.map.gz emd_43855_half_map_2.map.gz emd_43855_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43855 http://ftp.pdbj.org/pub/emdb/structures/EMD-43855 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43855 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43855 | HTTPS FTP |
-Validation report
| Summary document |  emd_43855_validation.pdf.gz emd_43855_validation.pdf.gz | 810.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43855_full_validation.pdf.gz emd_43855_full_validation.pdf.gz | 810.4 KB | Display | |
| Data in XML |  emd_43855_validation.xml.gz emd_43855_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_43855_validation.cif.gz emd_43855_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43855 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43855 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43855 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43855 | HTTPS FTP |
-Related structure data
| Related structure data |  9au5MC  9au8C  9au9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43855.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43855.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ternary complex of human DNA polymerase theta polymerase domain with a cognate C:G base pair | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map A
| File | emd_43855_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_43855_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of human DNA polymerase theta polymerase domain w...
| Entire | Name: Ternary complex of human DNA polymerase theta polymerase domain with a cognate C:G base pair |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of human DNA polymerase theta polymerase domain w...
| Supramolecule | Name: Ternary complex of human DNA polymerase theta polymerase domain with a cognate C:G base pair type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA polymerase theta
| Macromolecule | Name: DNA polymerase theta / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed DNA polymerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 89.585555 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GFKDNSPISD TSFSLQLSQD GLQLTPASSS SESLSIIDVA SDQNLFQTFI KEWRCKKRFS ISLACEKIRS LTSSKTATIG SRFKQASSP QEIPIRDDGF PIKGCDDTLV VGLAVCWGGR DAYYFSLQKE QKHSEISASL VPPSLDPSLT LKDRMWYLQS C LRKESDKE ...String: GFKDNSPISD TSFSLQLSQD GLQLTPASSS SESLSIIDVA SDQNLFQTFI KEWRCKKRFS ISLACEKIRS LTSSKTATIG SRFKQASSP QEIPIRDDGF PIKGCDDTLV VGLAVCWGGR DAYYFSLQKE QKHSEISASL VPPSLDPSLT LKDRMWYLQS C LRKESDKE CSVVIYDFIQ SYKILLLSCG ISLEQSYEDP KVACWLLDPD SQEPTLHSIV TSFLPHELPL LEGMETSQGI QS LGLNAGS EHSGRYRASV ESILIFNSMN QLNSLLQKEN LQDVFRKVEM PSQYCLALLE LNGIGFSTAE CESQKHIMQA KLD AIETQA YQLAGHSFSF TSSDDIAEVL FLELKLPPNR EMKNQGSKKT LGSTRRGIDN GRKLRLGRQF STSKDVLNKL KALH PLPGL ILEWRRITNA ITKVVFPLQR EKCLNPFLGM ERIYPVSQSH TATGRITFTE PNIQNVPRDF EIKMPTLVGE SPPSQ AVGK GLLPMGRGKY KKGFSVNPRC QAQMEERAAD RGMPFSISMR HAFVPFPGGS ILAADYSQLE LRILAHLSHD RRLIQV LNT GADVFRSIAA EWKMIEPESV GDDLRQQAKQ ICYGIIYGMG AKSLGEQMGI KENDAACYID SFKSRYTGIN QFMTETV KN CKRDGFVQTI LGRRRYLPGI KDNNPYRKAH AERQAINTIV QGSAADIVKI ATVNIQKQLE TFHSTFKSHG HREGMLQS D QTGLSRKRKL QGMFCPIRGG FFILQLHDEL LYEVAEEDVV QVAQIVKNEM ESAVKLSVKL KVKVKIGASW GELKDFDV UniProtKB: DNA polymerase theta |
-Macromolecule #2: DNA (5'-D(*AP*GP*CP*TP*CP*TP*AP*CP*GP*GP*AP*TP*GP*C)-3')
| Macromolecule | Name: DNA (5'-D(*AP*GP*CP*TP*CP*TP*AP*CP*GP*GP*AP*TP*GP*C)-3') type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 8.864712 KDa |
| Sequence | String: (DG)(DC)(DA)(DG)(DT)(DC)(DA)(DG)(DC)(DT) (DC)(DT)(DA)(DC)(DG)(DG)(DA)(DT)(DG)(DC) (DC)(DT)(DC)(DA)(DC)(DA)(DG)(DC)(DA) |
-Macromolecule #3: DNA (5'-D(P*GP*CP*AP*TP*CP*CP*GP*TP*AP*GP*(2DA))-3')
| Macromolecule | Name: DNA (5'-D(P*GP*CP*AP*TP*CP*CP*GP*TP*AP*GP*(2DA))-3') / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.174001 KDa |
| Sequence | String: (DT)(DG)(DC)(DT)(DG)(DT)(DG)(DA)(DG)(DG) (DC)(DA)(DT)(DC)(DC)(DG)(DT)(DA)(DG) (2DA) |
-Macromolecule #4: 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: DGT |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information | 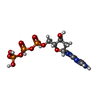 ChemComp-DGT: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Grid | Model: Quantifoil R1.2/1.3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.11 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 231192 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































