+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tau(291-407)-4E THF | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Tau / amyloid fibrils / PHF / QHF / STRUCTURAL PROTEIN | |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | El Mammeri N / Duan P / Hong M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: Milligram-scale assembly and NMR fingerprint of tau fibrils adopting the Alzheimer's disease fold. Authors: Pu Duan / Nadia El Mammeri / Mei Hong /  Abstract: In the Alzheimer's disease (AD) brain, the microtubule-associated protein tau aggregates into paired helical filaments in which each protofilament has a C-shaped conformation. In vitro assembly of ...In the Alzheimer's disease (AD) brain, the microtubule-associated protein tau aggregates into paired helical filaments in which each protofilament has a C-shaped conformation. In vitro assembly of tau fibrils adopting this fold is highly valuable for both fundamental and applied studies of AD without requiring patient-brain extracted fibrils. To date, reported methods for forming AD-fold tau fibrils have been irreproducible and sensitive to subtle variations in fibrillization conditions. Here, we describe a route to reproducibly assemble tau fibrils adopting the AD fold on the multi-milligram scale. We investigated the fibrillization conditions of two constructs and found that a tau (297-407) construct that contains four AD phospho-mimetic glutamate mutations robustly formed the C-shaped conformation. 2D and 3D correlation solid-state NMR spectra show a single predominant set of chemical shifts, indicating a single molecular conformation. Negative-stain electron microscopy and cryo-EM data confirm that the protofilament formed by 4E-tau (297-407) adopts the C-shaped conformation, which associates into paired, triple, and quadruple helical filaments. In comparison, NMR spectra indicate that a previously reported construct, tau (297-391), forms a mixture of a four-layered dimer structure and the C-shaped structure, whose populations are sensitive to the environmental conditions. The determination of the NMR chemical shifts of the AD-fold tau opens the possibility for future studies of tau fibril conformations and ligand binding by NMR. The quantitative assembly of tau fibrils adopting the AD fold should facilitate the development of diagnostic and therapeutic compounds that target AD tau. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43826.map.gz emd_43826.map.gz | 10.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43826-v30.xml emd-43826-v30.xml emd-43826.xml emd-43826.xml | 14.1 KB 14.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43826.png emd_43826.png | 48.8 KB | ||
| Masks |  emd_43826_msk_1.map emd_43826_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43826.cif.gz emd-43826.cif.gz | 3.8 KB | ||
| Others |  emd_43826_additional_1.map.gz emd_43826_additional_1.map.gz emd_43826_half_map_1.map.gz emd_43826_half_map_1.map.gz emd_43826_half_map_2.map.gz emd_43826_half_map_2.map.gz | 170.9 MB 171.2 MB 171.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43826 http://ftp.pdbj.org/pub/emdb/structures/EMD-43826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43826 | HTTPS FTP |
-Validation report
| Summary document |  emd_43826_validation.pdf.gz emd_43826_validation.pdf.gz | 886.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43826_full_validation.pdf.gz emd_43826_full_validation.pdf.gz | 886 KB | Display | |
| Data in XML |  emd_43826_validation.xml.gz emd_43826_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_43826_validation.cif.gz emd_43826_validation.cif.gz | 18.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43826 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43826 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43826 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43826 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43826.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43826.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43826_msk_1.map emd_43826_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
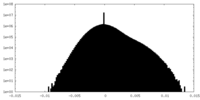
| Density Histograms |
-Additional map: #1
| File | emd_43826_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_43826_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_43826_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tau(297-407)-4E
| Entire | Name: Tau(297-407)-4E |
|---|---|
| Components |
|
-Supramolecule #1: Tau(297-407)-4E
| Supramolecule | Name: Tau(297-407)-4E / type: cell / ID: 1 / Parent: 0 / Details: tau construct with phospho-mimetic mutations |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 1.033 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 4.8 Å Applied symmetry - Helical parameters - Δ&Phi: -0.8 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 4.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 85048 |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Startup model | Type of model: NONE |
| Final angle assignment | Type: NOT APPLICABLE |
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)