[English] 日本語
 Yorodumi
Yorodumi- EMDB-43667: Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B (gB) in the postfusion conformation in complex with 1G2 and 7H3 Fabs | |||||||||
 Map data Map data | Sharpened | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | orthoherpesvirus / betaherpesvirus / cytomegalovirus / human betaherpesvirus 5 / human cytomegalovirus / HCMV / glycoprotein B / gB / HCMV gB / VIRAL PROTEIN / 7H3 / 1G2 / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell Golgi membrane / host cell endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / host cell plasma membrane / virion membrane / membrane Similarity search - Function | |||||||||
| Biological species |   Human betaherpesvirus 5 / Human betaherpesvirus 5 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
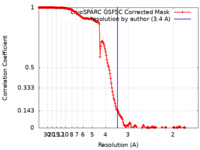
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Sponholtz MR / Byrne PO / McLellan JS | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Structure-based design of a soluble human cytomegalovirus glycoprotein B antigen stabilized in a prefusion-like conformation. Authors: Madeline R Sponholtz / Patrick O Byrne / Alison G Lee / Ajit R Ramamohan / Jory A Goldsmith / Ryan S McCool / Ling Zhou / Nicole V Johnson / Ching-Lin Hsieh / Megan Connors / Krithika P ...Authors: Madeline R Sponholtz / Patrick O Byrne / Alison G Lee / Ajit R Ramamohan / Jory A Goldsmith / Ryan S McCool / Ling Zhou / Nicole V Johnson / Ching-Lin Hsieh / Megan Connors / Krithika P Karthigeyan / Chelsea M Crooks / Adelaide S Fuller / John D Campbell / Sallie R Permar / Jennifer A Maynard / Dong Yu / Matthew J Bottomley / Jason S McLellan /  Abstract: Human cytomegalovirus (HCMV) glycoprotein B (gB) is a class III membrane fusion protein required for viral entry. HCMV vaccine candidates containing gB have demonstrated moderate clinical efficacy, ...Human cytomegalovirus (HCMV) glycoprotein B (gB) is a class III membrane fusion protein required for viral entry. HCMV vaccine candidates containing gB have demonstrated moderate clinical efficacy, but no HCMV vaccine has been approved. Here, we used structure-based design to identify and characterize amino acid substitutions that stabilize gB in its metastable prefusion conformation. One variant containing two engineered interprotomer disulfide bonds and two cavity-filling substitutions (gB-C7), displayed increased expression and thermostability. A 2.8 Å resolution cryoelectron microscopy structure shows that gB-C7 adopts a prefusion-like conformation, revealing additional structural elements at the membrane-distal apex. Unlike previous observations for several class I viral fusion proteins, mice immunized with postfusion or prefusion-stabilized forms of soluble gB protein displayed similar neutralizing antibody titers, here specifically against an HCMV laboratory strain on fibroblasts. Collectively, these results identify initial strategies to stabilize class III viral fusion proteins and provide tools to probe gB-directed antibody responses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43667.map.gz emd_43667.map.gz | 483.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43667-v30.xml emd-43667-v30.xml emd-43667.xml emd-43667.xml | 26.8 KB 26.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43667_fsc.xml emd_43667_fsc.xml | 17 KB | Display |  FSC data file FSC data file |
| Images |  emd_43667.png emd_43667.png | 43.3 KB | ||
| Masks |  emd_43667_msk_1.map emd_43667_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43667.cif.gz emd-43667.cif.gz | 7.7 KB | ||
| Others |  emd_43667_additional_1.map.gz emd_43667_additional_1.map.gz emd_43667_half_map_1.map.gz emd_43667_half_map_1.map.gz emd_43667_half_map_2.map.gz emd_43667_half_map_2.map.gz | 254 MB 475.5 MB 475.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43667 http://ftp.pdbj.org/pub/emdb/structures/EMD-43667 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43667 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43667 | HTTPS FTP |
-Related structure data
| Related structure data |  8vymMC  8vynC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43667.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43667.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8332 Å | ||||||||||||||||||||||||||||||||||||
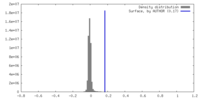
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43667_msk_1.map emd_43667_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
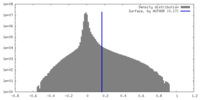
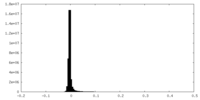
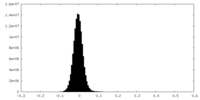
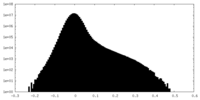
| Density Histograms |
-Additional map: Unsharpened
| File | emd_43667_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
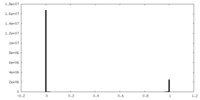
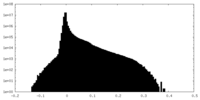
| Density Histograms |
-Half map: Half B
| File | emd_43667_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half B | ||||||||||||
| Projections & Slices |
| ||||||||||||
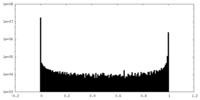
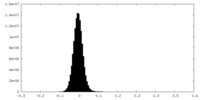
| Density Histograms |
-Half map: Half A
| File | emd_43667_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
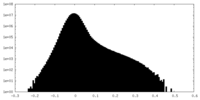
| Density Histograms |
- Sample components
Sample components
-Entire : Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B...
| Entire | Name: Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B (gB) in the postfusion conformation in complex with 1G2 and 7H3 Fabs |
|---|---|
| Components |
|
-Supramolecule #1: Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B...
| Supramolecule | Name: Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B (gB) in the postfusion conformation in complex with 1G2 and 7H3 Fabs type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|
-Macromolecule #1: Envelope glycoprotein B
| Macromolecule | Name: Envelope glycoprotein B / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human betaherpesvirus 5 Human betaherpesvirus 5 |
| Molecular weight | Theoretical: 88.931438 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MESRIWCLVV CVNLCIVCLG AAVSSSSTRG TSATHSHHSS HTTSAAHSRS GSVSQRVTSS QTVSHGVNET IYNTTLKYGD VVGVNTTKY PYRVCSMAQG TDLIRFERNI VCTSMKPINE DLDEGIMVVY KRNIVAHTFK VRVYQKVLTF RRSYAYIHTT Y LLGSNTEY ...String: MESRIWCLVV CVNLCIVCLG AAVSSSSTRG TSATHSHHSS HTTSAAHSRS GSVSQRVTSS QTVSHGVNET IYNTTLKYGD VVGVNTTKY PYRVCSMAQG TDLIRFERNI VCTSMKPINE DLDEGIMVVY KRNIVAHTFK VRVYQKVLTF RRSYAYIHTT Y LLGSNTEY VAPPMWEIHH INSHSQCYSS YSRVIAGTVF VAYHRDSYEN KTMQLMPDDY SNTHSTRYVT VKDQWHSRGS TW LYRETSN LNCMVTITTA RSKYPYHFFA TSTGDVVDIS PFYNGTNRNA SYFGENADKF FIFPNYTIVS DFGRPNSALE THR LVAFLE RADSVISWDI QDEKNVTCQL TFWEASERTI RSEAEDSYHF SSAKMTATFL SKKQEVNMSD SALDCVRDEA INKL QQIFN TSYNQTYEKY GNVSVFETTG GLVVFWQGIK QKSLVELERL ANRSSLNLTH NSTKSSTDGN NATHLSNMES VHNLV YAQL QFTYDTLRGY INRALAQIAE AWCVDQRRTL EVFKELSKIN PSAILSAIYN KPIAARFMGD VLGLASCVTI NQTSVK VLR DMNVKESPGR CYSRPVVIFN FANSSYVQYG QLGEDNEILL GNHRTEECQL PSLKIFIAGN SAYEYVDYLF KRMIDLS SI STVDSMIALD IDPLENTDFR VLELYSQKEL RSSNVFDLEE IMREFNSYKQ RVKYVEDKVV DPGSGYIPEA PRDGQAYV R KDGEWVLLST FLGAAASLEV LFQGPGHHHH HHHHSAWSHP QFEKGGASGG GGSGGSAWSH PQFEK UniProtKB: Envelope glycoprotein B |
-Macromolecule #2: 1G2 Fab Heavy Chain
| Macromolecule | Name: 1G2 Fab Heavy Chain / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.206172 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QLQLQESGPG LVKPSETLSL TCTVSGASID RSTYYWGWIR QPPGKGLEWI ANIYYNGRAV YSPSLKSRVT ISVDTSKNQF SLKVRSLTA ADTAVYYCAT RWNYFFDFDY WGRGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS ...String: QLQLQESGPG LVKPSETLSL TCTVSGASID RSTYYWGWIR QPPGKGLEWI ANIYYNGRAV YSPSLKSRVT ISVDTSKNQF SLKVRSLTA ADTAVYYCAT RWNYFFDFDY WGRGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS GVHTFPAVLQ SSGLYSLSSV VTVPSSSLGT QTYICNVNHK PSNTKVDKKV EPKSCD |
-Macromolecule #3: 1G2 Fab Light Chain
| Macromolecule | Name: 1G2 Fab Light Chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 22.853125 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVLTQPPSA SGTPGQRVTI SCSGSSSNIE TNYVSWYQQF PGTAPKLLIY RNNQRPSGVP DRFSGSKSGT SASLAISGLR SEDEAEYYC GTWDDNSWVF GGGTKLTVLG QPKAAPSVTL FPPSSEELQA NKATLVCLIS DFYPGAVTVA WKADSSPVKA G VETTTPSK ...String: QSVLTQPPSA SGTPGQRVTI SCSGSSSNIE TNYVSWYQQF PGTAPKLLIY RNNQRPSGVP DRFSGSKSGT SASLAISGLR SEDEAEYYC GTWDDNSWVF GGGTKLTVLG QPKAAPSVTL FPPSSEELQA NKATLVCLIS DFYPGAVTVA WKADSSPVKA G VETTTPSK QSNNKYAASS YLSLTPEQWK SHRSYSCQVT HEGSTVEKTV APTECS |
-Macromolecule #4: 7H3 Fab Light Chain
| Macromolecule | Name: 7H3 Fab Light Chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 22.879361 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVLSQPPSA SGTPGQRVTI SCSGSSSNIG KNYVYWYQQV PGTAPKLLMF KNNQRPSGVP DRFSGSKSGT SASLAISGLR SEDEADYYC SAWDGSLSRP LFGGGTKVTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP ...String: QSVLSQPPSA SGTPGQRVTI SCSGSSSNIG KNYVYWYQQV PGTAPKLLMF KNNQRPSGVP DRFSGSKSGT SASLAISGLR SEDEADYYC SAWDGSLSRP LFGGGTKVTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP SKQSNNKYAA SSYLSLTPEQ WKSHRSYSCQ VTHEGSTVEK TVAPTECS |
-Macromolecule #5: 7H3 Fab Heavy Chain
| Macromolecule | Name: 7H3 Fab Heavy Chain / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.152064 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLVQSGAE VKNPGASVKV SCKASGYTFT DYYIHWVRQA PGQGLEWMGW FNPNSGGTNF VQNFQGRVTM TRDTSISTAY MELSRLRSD DTAMYYCAKD SAKTASAYYG LNFFYYGMDV WGQGTTVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV K DYFPEPVT ...String: QVQLVQSGAE VKNPGASVKV SCKASGYTFT DYYIHWVRQA PGQGLEWMGW FNPNSGGTNF VQNFQGRVTM TRDTSISTAY MELSRLRSD DTAMYYCAKD SAKTASAYYG LNFFYYGMDV WGQGTTVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV K DYFPEPVT VSWNSGALTS GVHTFPAVLQ SSGLYSLSSV VTVPSSSLGT QTYICNVNHK PSNTKVDKKV EPKSCD |
-Macromolecule #8: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 8 / Number of copies: 10 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 2 mM Tris pH 8.0, 200 mM NaCl, 0.02% (w/v) sodium azide, 3% (v/v) glycerol, 0.25% CHAPS, and 0.1% (w/v) amphipol A8-35 |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | Soluble ectodomain of human cytomegalovirus (HCMV) glycoprotein B (gB) in the postfusion conformation, 1G2 Fab, and 7H3 Fab purified separately, mixed, and incubated for 30 minutes on ice prior to grid preparation |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 8679 / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)