[English] 日本語
 Yorodumi
Yorodumi- EMDB-43613: Structure of HamAB apo complex from the Escherichia coli Hachiman... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of HamAB apo complex from the Escherichia coli Hachiman defense system | |||||||||
 Map data Map data | Sharp map of the apo HamAB complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Nuclease-helicase complex / antiphage defense system / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
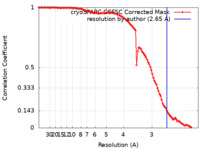
| Method | single particle reconstruction / cryo EM / Resolution: 2.65 Å | |||||||||
 Authors Authors | Tuck OT / Doudna JA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2024 Journal: bioRxiv / Year: 2024Title: Hachiman is a genome integrity sensor. Authors: Owen T Tuck / Benjamin A Adler / Emily G Armbruster / Arushi Lahiri / Jason J Hu / Julia Zhou / Joe Pogliano / Jennifer A Doudna /  Abstract: Hachiman is a broad-spectrum antiphage defense system of unknown function. We show here that Hachiman comprises a heterodimeric nuclease-helicase complex, HamAB. HamA, previously a protein of unknown ...Hachiman is a broad-spectrum antiphage defense system of unknown function. We show here that Hachiman comprises a heterodimeric nuclease-helicase complex, HamAB. HamA, previously a protein of unknown function, is the effector nuclease. HamB is the sensor helicase. HamB constrains HamA activity during surveillance of intact dsDNA. When the HamAB complex detects DNA damage, HamB helicase activity liberates HamA, unleashing nuclease activity. Hachiman activation degrades all DNA in the cell, creating 'phantom' cells devoid of both phage and host DNA. We demonstrate Hachiman activation in the absence of phage by treatment with DNA-damaging agents, suggesting that Hachiman responds to aberrant DNA states. Phylogenetic similarities between the Hachiman helicase and eukaryotic enzymes suggest this bacterial immune system has been repurposed for diverse functions across all domains of life. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43613.map.gz emd_43613.map.gz | 102.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43613-v30.xml emd-43613-v30.xml emd-43613.xml emd-43613.xml | 22.2 KB 22.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43613_fsc.xml emd_43613_fsc.xml | 10.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_43613.png emd_43613.png | 80.3 KB | ||
| Masks |  emd_43613_msk_1.map emd_43613_msk_1.map | 115.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43613.cif.gz emd-43613.cif.gz | 7 KB | ||
| Others |  emd_43613_additional_1.map.gz emd_43613_additional_1.map.gz emd_43613_half_map_1.map.gz emd_43613_half_map_1.map.gz emd_43613_half_map_2.map.gz emd_43613_half_map_2.map.gz | 58.5 MB 107.4 MB 107.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43613 http://ftp.pdbj.org/pub/emdb/structures/EMD-43613 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43613 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43613 | HTTPS FTP |
-Related structure data
| Related structure data |  8vx9MC  8vxaC  8vxcC  8vxyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43613.map.gz / Format: CCP4 / Size: 115.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43613.map.gz / Format: CCP4 / Size: 115.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharp map of the apo HamAB complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.048 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43613_msk_1.map emd_43613_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map of the apo HamAB complex
| File | emd_43613_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map of the apo HamAB complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A of the apo HamAB complex
| File | emd_43613_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A of the apo HamAB complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B of the apo HamAB complex
| File | emd_43613_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B of the apo HamAB complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Apo HamAB complex
| Entire | Name: Apo HamAB complex |
|---|---|
| Components |
|
-Supramolecule #1: Apo HamAB complex
| Supramolecule | Name: Apo HamAB complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 160 KDa |
-Macromolecule #1: HamA
| Macromolecule | Name: HamA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 28.923797 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MANFEDWCDS TERNISDHYL QSITARDAEC MFGVQVMAAL IPEHYASPRN IANAFEALGK PGLAAYIAGK LPETKQIRSG DLGEIFATE WINARSNGYK TPIKRLRWKD HRNMSMRGED VIGIYIDQSS QQLFFLKTEA KSRAKMTGEV VSEARDNLNK E QGLPSSHA ...String: MANFEDWCDS TERNISDHYL QSITARDAEC MFGVQVMAAL IPEHYASPRN IANAFEALGK PGLAAYIAGK LPETKQIRSG DLGEIFATE WINARSNGYK TPIKRLRWKD HRNMSMRGED VIGIYIDQSS QQLFFLKTEA KSRAKMTGEV VSEARDNLNK E QGLPSSHA LMFIADRLNE QGEELLAKAI LNATLRQGIV PGCVRHLIFL LSGNSSETML TTSIEKYTGQ NNQWGVCLRI AR HGEFIAA TFEKVISDAS NS UniProtKB: DUF1837 domain-containing protein |
-Macromolecule #2: HamB
| Macromolecule | Name: HamB / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 130.898453 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPATADEIIE AIKEASAVGF RGRLIARGQA RSVIWRDGDL PPDAPEFSAL LSQDLQGYAY ALIDLGLRLR ELNGDDAYAR IAFEQAGTA LESAIAKGKR DSRDTDFHFV MAAASYHLAH LSARAYSLLA MVGQDDNFSP IERALTQLIR RDLRTLRDNA L GFRLRGDG ...String: MPATADEIIE AIKEASAVGF RGRLIARGQA RSVIWRDGDL PPDAPEFSAL LSQDLQGYAY ALIDLGLRLR ELNGDDAYAR IAFEQAGTA LESAIAKGKR DSRDTDFHFV MAAASYHLAH LSARAYSLLA MVGQDDNFSP IERALTQLIR RDLRTLRDNA L GFRLRGDG SDVKITEILQ ARLNLPQDEN GDSESEEDIL FDGLDLALTD AYMSAISLYL LAVERGESRL LSRAIEKLRI SL SICAQFN MLPQWWLNFI TIHLLSDLWS DTFHERLPLV PVGGDAAEWP ALRELFIALL QRRPRAEIDL WPSQREAAGR SVN DNDDLV VSLPTSAGKT RIAELCILRC LAGGKRVVFI TPLRALSAQT EATLSRTFGP LGKTISMLYG SIGVSGMDED AIRQ RDIVV ATPEKLDFAL RNDPSIINDV GLFIFDEGHM IGADEREVRY EVQIQRLLRR QDADTRRIVC LSAILPDGEQ LDDFA GWLR RDKPGGPIKN NWRPTRLQFG EVIWSAPAGR LNLSVGYEAA WVSRFIVSRQ PPKVKLPNKK QRTKMFPSDN KELCLA TAW RLIEDGQTVL IYCPLRRSVE PFAETIVDLH QRGLLPSLFD AAPDILDTAI SLGEEWLGAH SPILACLRLG VALHHGA LP TAYRKEIERL LRDGVLKVTI SSPTLAQGLN LSATAIVMHS LHRNRELIKV SEFRNVIGRA GRAYVDVEGL VIYPIFDK V NKRQTNWHTL TSDTGAREME SGLIQLVCVL LIRMHTRLGG DLKALTEYVT NNAVAWEFPE IMTESPQERD IAQAIWEKQ LSTLDTAILS LLGENDIPDD QIETALDDIL QSSLWQRSLQ RYRDENERIL LKSGLLSRSR YIWQRSTAAG RRGYFLSGVG LTTGLRLDA IAAKANQLLI DANAAIMGGD AEEAIAAITA LAEEVFTFYP FIPDPLPGDW RGILRSWLLG EPMTNVANTQ A SETLQFVE NGLVYRLPWA MEAIRVRATA NGDLIGDTDT TLDDYELGFA VAAVETGTLS RSSSLLIQAG FSSRLAAIKV VT DTTADFQ SGQELRRWLN SEEVISHTDN HDWPTPETRV MWLEFLGSLS PKGSQVWSRH RYNGMVDWRD TPAVIGTPLQ LYT VDGIHH VLADDGTPLG SINGRINTNR RGLLRVEVDD ENGRAMFDYL GPDDFIST UniProtKB: DEAD/DEAH box helicase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: UltrAuFoil R2/2 / Material: GOLD / Mesh: 200 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 39.0 kPa | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 4796 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model / Details: v1.4.0 |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-8vx9: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)