+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a bacterial gasdermin small oval pore assembly | |||||||||
 Map data Map data | ~30mer oval pore | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | gasdermin / pore-forming protein / pyroptosis / bacteria / immunity / cell death / IMMUNE SYSTEM | |||||||||
| Function / homology | defense response to virus / plasma membrane / cytoplasm / Gasdermin bGSDM Function and homology information Function and homology information | |||||||||
| Biological species |  Vitiosangium sp. GDMCC 1.1324 (bacteria) Vitiosangium sp. GDMCC 1.1324 (bacteria) | |||||||||
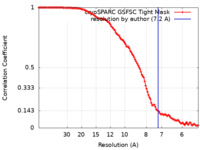
| Method | single particle reconstruction / cryo EM / Resolution: 7.2 Å | |||||||||
 Authors Authors | Johnson AG / Mayer ML / Kranzusch PJ | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structure and assembly of a bacterial gasdermin pore. Authors: Alex G Johnson / Megan L Mayer / Stefan L Schaefer / Nora K McNamara-Bordewick / Gerhard Hummer / Philip J Kranzusch /   Abstract: In response to pathogen infection, gasdermin (GSDM) proteins form membrane pores that induce a host cell death process called pyroptosis. Studies of human and mouse GSDM pores have revealed the ...In response to pathogen infection, gasdermin (GSDM) proteins form membrane pores that induce a host cell death process called pyroptosis. Studies of human and mouse GSDM pores have revealed the functions and architectures of assemblies comprising 24 to 33 protomers, but the mechanism and evolutionary origin of membrane targeting and GSDM pore formation remain unknown. Here we determine a structure of a bacterial GSDM (bGSDM) pore and define a conserved mechanism of pore assembly. Engineering a panel of bGSDMs for site-specific proteolytic activation, we demonstrate that diverse bGSDMs form distinct pore sizes that range from smaller mammalian-like assemblies to exceptionally large pores containing more than 50 protomers. We determine a cryo-electron microscopy structure of a Vitiosangium bGSDM in an active 'slinky'-like oligomeric conformation and analyse bGSDM pores in a native lipid environment to create an atomic-level model of a full 52-mer bGSDM pore. Combining our structural analysis with molecular dynamics simulations and cellular assays, our results support a stepwise model of GSDM pore assembly and suggest that a covalently bound palmitoyl can leave a hydrophobic sheath and insert into the membrane before formation of the membrane-spanning β-strand regions. These results reveal the diversity of GSDM pores found in nature and explain the function of an ancient post-translational modification in enabling programmed host cell death. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43508.map.gz emd_43508.map.gz | 75.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43508-v30.xml emd-43508-v30.xml emd-43508.xml emd-43508.xml | 14.9 KB 14.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43508_fsc.xml emd_43508_fsc.xml | 11.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_43508.png emd_43508.png | 43.8 KB | ||
| Masks |  emd_43508_msk_1.map emd_43508_msk_1.map | 149.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43508.cif.gz emd-43508.cif.gz | 5 KB | ||
| Others |  emd_43508_half_map_1.map.gz emd_43508_half_map_1.map.gz emd_43508_half_map_2.map.gz emd_43508_half_map_2.map.gz | 139.3 MB 139.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43508 http://ftp.pdbj.org/pub/emdb/structures/EMD-43508 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43508 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43508 | HTTPS FTP |
-Validation report
| Summary document |  emd_43508_validation.pdf.gz emd_43508_validation.pdf.gz | 742.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43508_full_validation.pdf.gz emd_43508_full_validation.pdf.gz | 741.7 KB | Display | |
| Data in XML |  emd_43508_validation.xml.gz emd_43508_validation.xml.gz | 19.7 KB | Display | |
| Data in CIF |  emd_43508_validation.cif.gz emd_43508_validation.cif.gz | 25.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43508 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43508 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43508 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43508 | HTTPS FTP |
-Related structure data
| Related structure data |  8sl0C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43508.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43508.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ~30mer oval pore | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.66 Å | ||||||||||||||||||||||||||||||||||||
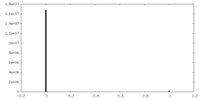
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43508_msk_1.map emd_43508_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
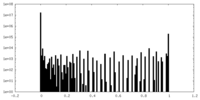
| Density Histograms |
-Half map: ~30mer oval pore half map B
| File | emd_43508_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ~30mer oval pore half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
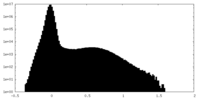
| Density Histograms |
-Half map: ~30mer oval pore half map A
| File | emd_43508_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ~30mer oval pore half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
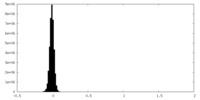
| Density Histograms |
- Sample components
Sample components
-Entire : Vitiosangium bacterial gasdermin
| Entire | Name: Vitiosangium bacterial gasdermin |
|---|---|
| Components |
|
-Supramolecule #1: Vitiosangium bacterial gasdermin
| Supramolecule | Name: Vitiosangium bacterial gasdermin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Vitiosangium sp. GDMCC 1.1324 (bacteria) Vitiosangium sp. GDMCC 1.1324 (bacteria) |
-Macromolecule #1: Vitiosangium bacterial gasdermin
| Macromolecule | Name: Vitiosangium bacterial gasdermin / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Vitiosangium sp. GDMCC 1.1324 (bacteria) Vitiosangium sp. GDMCC 1.1324 (bacteria) |
| Sequence | String: SGLCSDPAIT YLKRLGYNVV RLPREGIQPL HLLGQQRGTV EYLGSLEKLI TQPPSEPPAI TRDQAAAGIN GQKTENLSFS IGINILKSVL AQFGAGAGIE AQYNQARKVR FEFSNVLADS VEPLAVGQFL KMAEVDADNP VLKQYVLGNG RLYVITQVIK SNEFTVAAEK ...String: SGLCSDPAIT YLKRLGYNVV RLPREGIQPL HLLGQQRGTV EYLGSLEKLI TQPPSEPPAI TRDQAAAGIN GQKTENLSFS IGINILKSVL AQFGAGAGIE AQYNQARKVR FEFSNVLADS VEPLAVGQFL KMAEVDADNP VLKQYVLGNG RLYVITQVIK SNEFTVAAEK SGGGSIQLDV PEIQKVVGGK LKVEASVSSQ STVTYKGEKQ LVFGFKCFEI GVKNGEITLF ASQLVPR UniProtKB: Gasdermin bGSDM |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 150 mM NaCl, 20 mM HEPES-HOH (pH 7.5), 50 mM HECAMEG | ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | The sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 33411 / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)