+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Phosphorylated human NCC in complex with indapamide | |||||||||
 Map data Map data | Phosphorylated human NCC in complex with indapamide | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Phosphorylation / a thiazide-like drug / Indapamide / Outward-open / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsodium:chloride symporter activity / apical plasma membrane / ATP binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Zhao YX / Cao EH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural bases for Na-Cl cotransporter inhibition by thiazide diuretic drugs and activation by kinases. Authors: Yongxiang Zhao / Heidi Schubert / Alan Blakely / Biff Forbush / Micholas Dean Smith / Jesse Rinehart / Erhu Cao /   Abstract: The Na-Cl cotransporter (NCC) drives salt reabsorption in the kidney and plays a decisive role in balancing electrolytes and blood pressure. Thiazide and thiazide-like diuretics inhibit NCC-mediated ...The Na-Cl cotransporter (NCC) drives salt reabsorption in the kidney and plays a decisive role in balancing electrolytes and blood pressure. Thiazide and thiazide-like diuretics inhibit NCC-mediated renal salt retention and have been cornerstones for treating hypertension and edema since the 1950s. Here we determine NCC co-structures individually complexed with the thiazide drug hydrochlorothiazide, and two thiazide-like drugs chlorthalidone and indapamide, revealing that they fit into an orthosteric site and occlude the NCC ion translocation pathway. Aberrant NCC activation by the WNKs-SPAK kinase cascade underlies Familial Hyperkalemic Hypertension, but it remains unknown whether/how phosphorylation transforms the NCC structure to accelerate ion translocation. We show that an intracellular amino-terminal motif of NCC, once phosphorylated, associates with the carboxyl-terminal domain, and together, they interact with the transmembrane domain. These interactions suggest a phosphorylation-dependent allosteric network that directly influences NCC ion translocation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43411.map.gz emd_43411.map.gz | 197.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43411-v30.xml emd-43411-v30.xml emd-43411.xml emd-43411.xml | 22.2 KB 22.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43411.png emd_43411.png | 41.4 KB | ||
| Filedesc metadata |  emd-43411.cif.gz emd-43411.cif.gz | 7 KB | ||
| Others |  emd_43411_additional_1.map.gz emd_43411_additional_1.map.gz emd_43411_half_map_1.map.gz emd_43411_half_map_1.map.gz emd_43411_half_map_2.map.gz emd_43411_half_map_2.map.gz | 194.1 MB 194.2 MB 194.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43411 http://ftp.pdbj.org/pub/emdb/structures/EMD-43411 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43411 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43411 | HTTPS FTP |
-Related structure data
| Related structure data |  8vpnMC  8vppC  9bwtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43411.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43411.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Phosphorylated human NCC in complex with indapamide | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Additional Map
| File | emd_43411_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Additional Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map A
| File | emd_43411_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_43411_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Phosphorylated human NCC in complex with Indapamide
| Entire | Name: Phosphorylated human NCC in complex with Indapamide |
|---|---|
| Components |
|
-Supramolecule #1: Phosphorylated human NCC in complex with Indapamide
| Supramolecule | Name: Phosphorylated human NCC in complex with Indapamide / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 12 member 3
| Macromolecule | Name: Solute carrier family 12 member 3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 113.364648 KDa |
| Recombinant expression | Organism: Mammalian expression vector BsrGI-MCS-pcDNA3.1 (others) |
| Sequence | String: MAELPTTETP GDATLCSGRF TISTLLSSDE PSPPAAYDSS HPSHLTHSST FCMR(TPO)FGYN(TPO) IDVVPTYEHY AN (SEP)TQPGEP RKVRPTLADL HSFLKEGRHL HALAFDSRPS HEMTDGLVEG EAGTSSEKNP EEPVRFGWVK GVMIRCMLN IWGVILYLRL ...String: MAELPTTETP GDATLCSGRF TISTLLSSDE PSPPAAYDSS HPSHLTHSST FCMR(TPO)FGYN(TPO) IDVVPTYEHY AN (SEP)TQPGEP RKVRPTLADL HSFLKEGRHL HALAFDSRPS HEMTDGLVEG EAGTSSEKNP EEPVRFGWVK GVMIRCMLN IWGVILYLRL PWITAQAGIV LTWIIILLSV TVTSITGLSI SAISTNGKVK SGGTYFLISR SLGPELGGSI GLIFAFANAV GVAMHTVGF AETVRDLLQE YGAPIVDPIN DIRIIAVVSV TVLLAISLAG MEWESKAQVL FFLVIMVSFA NYLVGTLIPP S EDKASKGF FSYRADIFVQ NLVPDWRGPD GTFFGMFSIF FPSATGILAG ANISGDLKDP AIAIPKGTLM AIFWTTISYL AI SATIGSC VVRDASGVLN DTVTPGWGAC EGLACSYGWN FTECTQQHSC HYGLINYYQT MSMVSGFAPL ITAGIFGATL SSA LACLVS AAKVFQCLCE DQLYPLIGFF GKGYGKNKEP VRGYLLAYAI AVAFIIIAEL NTIAPIISNF FLCSYALINF SCFH ASITN SPGWRPSFQY YNKWAALFGA IISVVIMFLL TWWAALIAIG VVLFLLLYVI YKKPEVNWGS SVQAGSYNLA LSYSV GLNE VEDHIKNYRP QCLVLTGPPN FRPALVDFVG TFTRNLSLMI CGHVLIGPHK QRMPELQLIA NGHTKWLNKR KIKAFY SDV IAEDLRRGVQ ILMQAAGLGR MKPNILVVGF KKNWQSAHPA TVEDYIGILH DAFDFNYGVC VMRMREGLNV SKMMQAH IN PVFDPAEDGK EASARVDPKA LVKEEQATTI FQSEQGKKTI DIYWLFDDGG LTLLIPYLLG RKRRWSKCKI RVFVGGQI N RMDQERKAII SLLSKFRLGF HEVHILPDIN QNPRAEHTKR FEDMIAPFRL NDGFKDEATV NEMRRDCPWK ISDEEITKN RVKSLRQVRL NEIVLDYSRD AALIVITLPI GRKGKCPSSL YMAWLETLSQ DLRPPVILIR GNQENVLTFY CQ UniProtKB: Solute carrier family 12 member 3 |
-Macromolecule #2: Solute carrier family 12 member 3
| Macromolecule | Name: Solute carrier family 12 member 3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 113.124711 KDa |
| Recombinant expression | Organism: Mammalian expression vector BsrGI-MCS-pcDNA3.1 (others) |
| Sequence | String: MAELPTTETP GDATLCSGRF TISTLLSSDE PSPPAAYDSS HPSHLTHSST FCMRTFGYNT IDVVPTYEHY ANSTQPGEPR KVRPTLADL HSFLKEGRHL HALAFDSRPS HEMTDGLVEG EAGTSSEKNP EEPVRFGWVK GVMIRCMLNI WGVILYLRLP W ITAQAGIV ...String: MAELPTTETP GDATLCSGRF TISTLLSSDE PSPPAAYDSS HPSHLTHSST FCMRTFGYNT IDVVPTYEHY ANSTQPGEPR KVRPTLADL HSFLKEGRHL HALAFDSRPS HEMTDGLVEG EAGTSSEKNP EEPVRFGWVK GVMIRCMLNI WGVILYLRLP W ITAQAGIV LTWIIILLSV TVTSITGLSI SAISTNGKVK SGGTYFLISR SLGPELGGSI GLIFAFANAV GVAMHTVGFA ET VRDLLQE YGAPIVDPIN DIRIIAVVSV TVLLAISLAG MEWESKAQVL FFLVIMVSFA NYLVGTLIPP SEDKASKGFF SYR ADIFVQ NLVPDWRGPD GTFFGMFSIF FPSATGILAG ANISGDLKDP AIAIPKGTLM AIFWTTISYL AISATIGSCV VRDA SGVLN DTVTPGWGAC EGLACSYGWN FTECTQQHSC HYGLINYYQT MSMVSGFAPL ITAGIFGATL SSALACLVSA AKVFQ CLCE DQLYPLIGFF GKGYGKNKEP VRGYLLAYAI AVAFIIIAEL NTIAPIISNF FLCSYALINF SCFHASITNS PGWRPS FQY YNKWAALFGA IISVVIMFLL TWWAALIAIG VVLFLLLYVI YKKPEVNWGS SVQAGSYNLA LSYSVGLNEV EDHIKNY RP QCLVLTGPPN FRPALVDFVG TFTRNLSLMI CGHVLIGPHK QRMPELQLIA NGHTKWLNKR KIKAFYSDVI AEDLRRGV Q ILMQAAGLGR MKPNILVVGF KKNWQSAHPA TVEDYIGILH DAFDFNYGVC VMRMREGLNV SKMMQAHINP VFDPAEDGK EASARVDPKA LVKEEQATTI FQSEQGKKTI DIYWLFDDGG LTLLIPYLLG RKRRWSKCKI RVFVGGQINR MDQERKAIIS LLSKFRLGF HEVHILPDIN QNPRAEHTKR FEDMIAPFRL NDGFKDEATV NEMRRDCPWK ISDEEITKNR VKSLRQVRLN E IVLDYSRD AALIVITLPI GRKGKCPSSL YMAWLETLSQ DLRPPVILIR GNQENVLTFY CQ UniProtKB: Solute carrier family 12 member 3 |
-Macromolecule #3: 4-chloro-N-[(2S)-2-methyl-2,3-dihydro-1H-indol-1-yl]-3-sulfamoylb...
| Macromolecule | Name: 4-chloro-N-[(2S)-2-methyl-2,3-dihydro-1H-indol-1-yl]-3-sulfamoylbenzamide type: ligand / ID: 3 / Number of copies: 1 / Formula: BL1 |
|---|---|
| Molecular weight | Theoretical: 365.835 Da |
| Chemical component information | 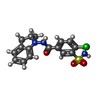 ChemComp-BL1: |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 5 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)












































