[English] 日本語
 Yorodumi
Yorodumi- EMDB-43114: Kinetic intermediate states of HIV-1 RT DNA synthesis captured by... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Kinetic intermediate states of HIV-1 RT DNA synthesis captured by cryo-EM | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Reverse Transcription / Time-resolved / HIV-1 / Cryo-EM / TRANSCRIPTION / TRANSFERASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / synthetic construct (others) Human immunodeficiency virus 1 / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Vergara S / Zhou X / Santiago U / Conway JF / Sluis-Cremer N / Calero G | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis of deoxynucleotide addition by HIV-1 RT during reverse transcription. Authors: Sandra Vergara / Xiaohong Zhou / Ulises Santiago / Mounia Alaoui-El-Azher / James F Conway / Nicolas Sluis-Cremer / Guillermo Calero /  Abstract: Reverse transcription of the retroviral RNA genome into DNA is an integral step during HIV-1 replication. Despite a wealth of structural information on reverse transcriptase (RT), we lack insight ...Reverse transcription of the retroviral RNA genome into DNA is an integral step during HIV-1 replication. Despite a wealth of structural information on reverse transcriptase (RT), we lack insight into the intermediate states of DNA synthesis. Using catalytically active substrates, and a blot/diffusion cryo-electron microscopy approach, we capture 11 structures encompassing reactant, intermediate and product states of dATP addition by RT at 2.2 to 3.0 Å resolution. In the reactant state, dATP binding to RT-template/primer involves a single Mg (site B) inducing formation of a negatively charged pocket where a second floating Mg can bind (site A). During the intermediate state, the α-phosphate oxygen from a previously unobserved dATP conformer aligns with site A Mg and the primer 3'-OH for nucleophilic attack. The product state, comprises two substrate conformations including an incorporated dAMP with the pyrophosphate leaving group coordinated by metal B and stabilized through H-bonds. Moreover, K220 mutants significantly impact the rate of dNTP incorporation by RT and HIV-1 replication capacity. This work sheds light into the dynamic components of a reaction that is central to HIV-1 replication. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43114.map.gz emd_43114.map.gz | 108.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43114-v30.xml emd-43114-v30.xml emd-43114.xml emd-43114.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43114_fsc.xml emd_43114_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_43114.png emd_43114.png | 98.1 KB | ||
| Masks |  emd_43114_msk_1.map emd_43114_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43114.cif.gz emd-43114.cif.gz | 6.5 KB | ||
| Others |  emd_43114_half_map_1.map.gz emd_43114_half_map_1.map.gz emd_43114_half_map_2.map.gz emd_43114_half_map_2.map.gz | 200.2 MB 200.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43114 http://ftp.pdbj.org/pub/emdb/structures/EMD-43114 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43114 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43114 | HTTPS FTP |
-Validation report
| Summary document |  emd_43114_validation.pdf.gz emd_43114_validation.pdf.gz | 884.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43114_full_validation.pdf.gz emd_43114_full_validation.pdf.gz | 884.3 KB | Display | |
| Data in XML |  emd_43114_validation.xml.gz emd_43114_validation.xml.gz | 21.6 KB | Display | |
| Data in CIF |  emd_43114_validation.cif.gz emd_43114_validation.cif.gz | 27.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43114 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43114 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43114 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43114 | HTTPS FTP |
-Related structure data
| Related structure data |  8vb6MC  8vb7C  8vb8C  8vb9C  8vbcC  8vbdC  8vbeC  8vbfC  8vbgC  8vbhC  8vbiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43114.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43114.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43114_msk_1.map emd_43114_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
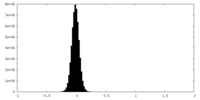
| Density Histograms |
-Half map: #2
| File | emd_43114_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
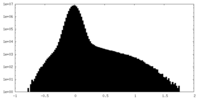
| Density Histograms |
-Half map: #1
| File | emd_43114_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Reverse transcriptase/ribonuclease H in complex with DNA aptamer
| Entire | Name: Reverse transcriptase/ribonuclease H in complex with DNA aptamer |
|---|---|
| Components |
|
-Supramolecule #1: Reverse transcriptase/ribonuclease H in complex with DNA aptamer
| Supramolecule | Name: Reverse transcriptase/ribonuclease H in complex with DNA aptamer type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Macromolecule #1: HIV-1 reverse transcriptase/ribonuclease H P66 subunit
| Macromolecule | Name: HIV-1 reverse transcriptase/ribonuclease H P66 subunit type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 64.104457 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVPISPIETV PVKLKPGMDG PKVKQWPLTE EKIKALVEIC TEMEKEGKIS KIGPENPYNT PVFAIKKKDS TKWRKLVDFR ELNKRTQDF WEVQLGIPHP AGLKKKKSVT VLDVGDAYFS VPLDEDFRKY TAFTIPSINN ETPGIRYQYN VLPQGWKGSP A IFQSSMTK ...String: MVPISPIETV PVKLKPGMDG PKVKQWPLTE EKIKALVEIC TEMEKEGKIS KIGPENPYNT PVFAIKKKDS TKWRKLVDFR ELNKRTQDF WEVQLGIPHP AGLKKKKSVT VLDVGDAYFS VPLDEDFRKY TAFTIPSINN ETPGIRYQYN VLPQGWKGSP A IFQSSMTK ILEPFKKQNP DIVIYQYMDD LYVGSDLEIG QHRTKIEELR QHLLRWGLTT PDKKHQKEPP FLWMGYELHP DK WTVQPIV LPEKDSWTVN DIQKLVGKLN WASQIYPGIK VRQLSKLLRG TKALTEVIPL TEEAELELAE NREILKEPVH GVY YDPSKD LIAEIQKQGQ GQWTYQIYQE PFKNLKTGKY ARMRGAHTND VKQLTEAVQK ITTESIVIWG KTPKFKLPIQ KETW ETWWT EYWQATWIPE WEFVNTPPLV KLWYQLEKEP IVGAETFYVD GAANRETKLG KAGYVTNKGR QKVVPLTNTT NQKTE LQAI YLALQDSGLE VNIVTNSQYA LGIIQAQPDK SESELVNQII EQLIKKEKVY LAWVPAHKGI GGNEQVDKLV SAG UniProtKB: Gag-Pol polyprotein |
-Macromolecule #2: HIV-1 reverse transcriptase P51 subunit
| Macromolecule | Name: HIV-1 reverse transcriptase P51 subunit / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 51.944691 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAHHHHHHAL EVLFQGPISP IETVPVKLKP GMDGPKVKQW PLTEEKIKAL VEICTEMEKE GKISKIGPEN PYNTPVFAIK KKDSTKWRK LVDFRELNKR TQDFWEVQLG IPHPAGLKKK KSVTVLDVGD AYFSVPLDED FRKYTAFTIP SINNETPGIR Y QYNVLPQG ...String: MAHHHHHHAL EVLFQGPISP IETVPVKLKP GMDGPKVKQW PLTEEKIKAL VEICTEMEKE GKISKIGPEN PYNTPVFAIK KKDSTKWRK LVDFRELNKR TQDFWEVQLG IPHPAGLKKK KSVTVLDVGD AYFSVPLDED FRKYTAFTIP SINNETPGIR Y QYNVLPQG WKGSPAIFQS SMTKILEPFK KQNPDIVIYQ YMDDLYVGSD LEIGQHRTKI EELRQHLLRW GLTTPDKKHQ KE PPFLWMG YELHPDKWTV QPIVLPEKDS WTVNDIQKLV GKLNWASQIY PGIKVRQLCK LLRGTKALTE VIPLTEEAEL ELA ENREIL KEPVHGVYYD PSKDLIAEIQ KQGQGQWTYQ IYQEPFKNLK TGKYARMRGA HTNDVKQLTE AVQKITTESI VIWG KTPKF KLPIQKETWE TWWTEYWQAT WIPEWEFVNT PPLVKLWYQ UniProtKB: Gag-Pol polyprotein |
-Macromolecule #3: DNA (38-MER)
| Macromolecule | Name: DNA (38-MER) / type: dna / ID: 3 / Details: DNA aptamer / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 11.739513 KDa |
| Sequence | String: (DT)(DA)(DA)(DT)(DT)(DC)(OMC)(DC)(OMC)(DC) (DC)(DC)(DT)(DT)(DC)(DG)(DG)(DT)(DG) (DC)(DT)(DT)(DT)(DG)(DC)(DA)(DC)(DC)(DG) (DA)(DA)(DG)(DG)(DG)(DG)(DG)(DG)(DG) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Material: GOLD / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.25 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8vb6: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)