+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Computational Designed Nanocage O43_129 | |||||||||
 Map data Map data | Main Map, DeepEMhancer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | De Novo / Nanocage / O43_129 / extendable nanomaterials / DE NOVO PROTEIN | |||||||||
| Biological species | unidentified (others) / synthetic construct (others) | |||||||||
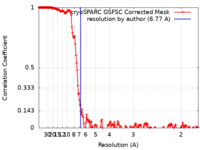
| Method | single particle reconstruction / cryo EM / Resolution: 6.77 Å | |||||||||
 Authors Authors | Weidle C / Kibler RD | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Blueprinting extendable nanomaterials with standardized protein blocks. Authors: Timothy F Huddy / Yang Hsia / Ryan D Kibler / Jinwei Xu / Neville Bethel / Deepesh Nagarajan / Rachel Redler / Philip J Y Leung / Connor Weidle / Alexis Courbet / Erin C Yang / Asim K Bera / ...Authors: Timothy F Huddy / Yang Hsia / Ryan D Kibler / Jinwei Xu / Neville Bethel / Deepesh Nagarajan / Rachel Redler / Philip J Y Leung / Connor Weidle / Alexis Courbet / Erin C Yang / Asim K Bera / Nicolas Coudray / S John Calise / Fatima A Davila-Hernandez / Hannah L Han / Kenneth D Carr / Zhe Li / Ryan McHugh / Gabriella Reggiano / Alex Kang / Banumathi Sankaran / Miles S Dickinson / Brian Coventry / T J Brunette / Yulai Liu / Justas Dauparas / Andrew J Borst / Damian Ekiert / Justin M Kollman / Gira Bhabha / David Baker /   Abstract: A wooden house frame consists of many different lumber pieces, but because of the regularity of these building blocks, the structure can be designed using straightforward geometrical principles. The ...A wooden house frame consists of many different lumber pieces, but because of the regularity of these building blocks, the structure can be designed using straightforward geometrical principles. The design of multicomponent protein assemblies, in comparison, has been much more complex, largely owing to the irregular shapes of protein structures. Here we describe extendable linear, curved and angled protein building blocks, as well as inter-block interactions, that conform to specified geometric standards; assemblies designed using these blocks inherit their extendability and regular interaction surfaces, enabling them to be expanded or contracted by varying the number of modules, and reinforced with secondary struts. Using X-ray crystallography and electron microscopy, we validate nanomaterial designs ranging from simple polygonal and circular oligomers that can be concentrically nested, up to large polyhedral nanocages and unbounded straight 'train track' assemblies with reconfigurable sizes and geometries that can be readily blueprinted. Because of the complexity of protein structures and sequence-structure relationships, it has not previously been possible to build up large protein assemblies by deliberate placement of protein backbones onto a blank three-dimensional canvas; the simplicity and geometric regularity of our design platform now enables construction of protein nanomaterials according to 'back of an envelope' architectural blueprints. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42906.map.gz emd_42906.map.gz | 554.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42906-v30.xml emd-42906-v30.xml emd-42906.xml emd-42906.xml | 23.1 KB 23.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42906_fsc.xml emd_42906_fsc.xml | 18.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_42906.png emd_42906.png | 188.2 KB | ||
| Filedesc metadata |  emd-42906.cif.gz emd-42906.cif.gz | 6.7 KB | ||
| Others |  emd_42906_additional_1.map.gz emd_42906_additional_1.map.gz emd_42906_half_map_1.map.gz emd_42906_half_map_1.map.gz emd_42906_half_map_2.map.gz emd_42906_half_map_2.map.gz | 300 MB 575.8 MB 575.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42906 http://ftp.pdbj.org/pub/emdb/structures/EMD-42906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42906 | HTTPS FTP |
-Related structure data
| Related structure data |  8v2dMC  8g9jC  8g9kC  8ga6C  8ga7C  8gelC  8tl7C  8v3bC C: citing same article ( M: atomic model generated by this map |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42906.map.gz / Format: CCP4 / Size: 620.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42906.map.gz / Format: CCP4 / Size: 620.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main Map, DeepEMhancer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.885 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Homogeneous Refinement
| File | emd_42906_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Homogeneous Refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map A
| File | emd_42906_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_42906_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Nanocage O43_129
| Entire | Name: Nanocage O43_129 |
|---|---|
| Components |
|
-Supramolecule #1: Nanocage O43_129
| Supramolecule | Name: Nanocage O43_129 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Chain A and Chain B are expressed separately in E coli. Complex is formed by mixing the cell lysis of both expressions, forming the nanocage. Nanocage was purified through a His tag on chain A. |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Molecular weight | Theoretical: 1.60 MDa |
-Macromolecule #1: O43_129 component B
| Macromolecule | Name: O43_129 component B / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 34.478918 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGDDLLLKLL ELLVEQARVS AEFARRQGDE KMLEEVARKA EEVARKAESI ARKARKEGNL ELALKALEIL VRAAHVLAEI ARERGNEEL QKKAHKLAKE ALRQVIEIAI RAIQEGNLEL AIIALHISVR IAEVLLETRP DDREEIREQQ AIFELLIAAL E AAIRLEKL ...String: MGDDLLLKLL ELLVEQARVS AEFARRQGDE KMLEEVARKA EEVARKAESI ARKARKEGNL ELALKALEIL VRAAHVLAEI ARERGNEEL QKKAHKLAKE ALRQVIEIAI RAIQEGNLEL AIIALHISVR IAEVLLETRP DDREEIREQQ AIFELLIAAL E AAIRLEKL KEEGAPPEQI ERVAEHGLER LKEIAKEISK EVDSPESKRI AYKIVAAAAE FLLKILAEGG ATPEQLERVT EH ALEVLKE VAKELADSPE SGLAALAAIA SLAKLGLEQL KEIGAPPEQQ RRVTKAGIEA VREIYRYGRK LY |
-Macromolecule #2: O43_129 component A
| Macromolecule | Name: O43_129 component A / type: protein_or_peptide / ID: 2 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 33.976965 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCDAIQAAA ALGEAGISSN EILELLAAAA ELGLDPDAIQ AAAQLGEAGI SSEEIKELLR AAHELGLDPD AIAAAADLGQ AGVSPVEIL ALLIAASVLG LDPDAIQAAA ALGEAGISAE EIIELLTAAR DLGLDPDAIQ AAAQLGEAGI SSEEIKELLR A AHELGLDP ...String: MGCDAIQAAA ALGEAGISSN EILELLAAAA ELGLDPDAIQ AAAQLGEAGI SSEEIKELLR AAHELGLDPD AIAAAADLGQ AGVSPVEIL ALLIAASVLG LDPDAIQAAA ALGEAGISAE EIIELLTAAR DLGLDPDAIQ AAAQLGEAGI SSEEIKELLR A AHELGLDP DCIAAAADLG QAGISSSEIT ALLLAAAAIE LAKRADDKDV REIVRDALEL ASRSTNDEVI RLALEAAVLA AR STDSDVL EIVKDALELA KQSTNEEVIK LALKAAVLAA KSTDEEVLEE VKEALRRAKE STDEEEIKEE LRKAVEEAEG GSW LEHHHH HH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 25mM Tris HCl, 300mM NaCl, pH 8 | |||||||||
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.9 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: Other / Chain - Initial model type: in silico model / Details: De Novo Design |
|---|---|
| Details | Model was initially fit in Chimera. Model was further refined with Namdinator, Isolde, and Coot |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 955.4 |
| Output model |  PDB-8v2d: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)