+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4270 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Chloroplast F1Fo conformation 1 | |||||||||
 Map data Map data | chloroplast F1Fo conformation 1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP synthase / membrane protein complex / molecular motor / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationphotosynthetic electron transport in photosystem I / photosynthetic electron transport in photosystem II / proton motive force-driven ATP synthesis / chloroplast thylakoid membrane / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism ...photosynthetic electron transport in photosystem I / photosynthetic electron transport in photosystem II / proton motive force-driven ATP synthesis / chloroplast thylakoid membrane / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / lipid binding / ATP hydrolysis activity / mitochondrion / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Spinacia oleracea (spinach) Spinacia oleracea (spinach) | |||||||||
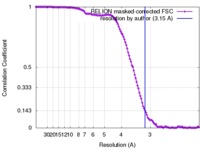
| Method | single particle reconstruction / cryo EM / Resolution: 3.15 Å | |||||||||
 Authors Authors | Hahn A / Vonck J | |||||||||
 Citation Citation |  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Structure, mechanism, and regulation of the chloroplast ATP synthase. Authors: Alexander Hahn / Janet Vonck / Deryck J Mills / Thomas Meier / Werner Kühlbrandt /  Abstract: The chloroplast adenosine triphosphate (ATP) synthase uses the electrochemical proton gradient generated by photosynthesis to produce ATP, the energy currency of all cells. Protons conducted through ...The chloroplast adenosine triphosphate (ATP) synthase uses the electrochemical proton gradient generated by photosynthesis to produce ATP, the energy currency of all cells. Protons conducted through the membrane-embedded F motor drive ATP synthesis in the F head by rotary catalysis. We determined the high-resolution structure of the complete cFF complex by cryo-electron microscopy, resolving side chains of all 26 protein subunits, the five nucleotides in the F head, and the proton pathway to and from the rotor ring. The flexible peripheral stalk redistributes differences in torsional energy across three unequal steps in the rotation cycle. Plant ATP synthase is autoinhibited by a β-hairpin redox switch in subunit γ that blocks rotation in the dark. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4270.map.gz emd_4270.map.gz | 153.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4270-v30.xml emd-4270-v30.xml emd-4270.xml emd-4270.xml | 27.5 KB 27.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4270_fsc.xml emd_4270_fsc.xml | 12.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_4270.png emd_4270.png | 158.8 KB | ||
| Masks |  emd_4270_msk_1.map emd_4270_msk_1.map | 163.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4270.cif.gz emd-4270.cif.gz | 7.4 KB | ||
| Others |  emd_4270_half_map_1.map.gz emd_4270_half_map_1.map.gz emd_4270_half_map_2.map.gz emd_4270_half_map_2.map.gz | 129.3 MB 129.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4270 http://ftp.pdbj.org/pub/emdb/structures/EMD-4270 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4270 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4270 | HTTPS FTP |
-Related structure data
| Related structure data |  6fkfMC  4271C  4272C  4273C  6fkhC  6fkiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4270.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4270.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | chloroplast F1Fo conformation 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.053 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4270_msk_1.map emd_4270_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_4270_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_4270_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Chloroplast ATP synthase
+Supramolecule #1: Chloroplast ATP synthase
+Macromolecule #1: ATP synthase subunit alpha, chloroplastic
+Macromolecule #2: ATP synthase subunit beta, chloroplastic
+Macromolecule #3: ATP synthase gamma chain, chloroplastic
+Macromolecule #4: ATP synthase delta chain, chloroplastic
+Macromolecule #5: ATP synthase subunit b', chloroplastic
+Macromolecule #6: ATP synthase subunit b, chloroplastic
+Macromolecule #7: ATP synthase subunit a, chloroplastic
+Macromolecule #8: ATP synthase subunit c, chloroplastic
+Macromolecule #9: ATP synthase epsilon chain, chloroplastic
+Macromolecule #10: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #11: MAGNESIUM ION
+Macromolecule #12: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #13: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 70 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number real images: 6254 / Average exposure time: 62.0 sec. / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.5 µm / Calibrated defocus min: 1.5 µm / Calibrated magnification: 132953 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | real space refinement |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
| Output model |  PDB-6fkf: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)