+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Site-one protease without SPRING | |||||||||
 Map data Map data | Primary, unsharpened map for S1P | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | serine protease cholesterol metabolism zymogen activation Protein complex glycoprotein secretory pathway / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsite-1 protease / CREB3 factors activate genes / ATF6-mediated unfolded protein response / regulation of cholesterol biosynthetic process / ATF6 (ATF6-alpha) activates chaperones / ATF6B (ATF6-beta) activates chaperones / Assembly of active LPL and LIPC lipase complexes / membrane protein intracellular domain proteolysis / regulation of vesicle-mediated transport / Regulation of cholesterol biosynthesis by SREBP (SREBF) ...site-1 protease / CREB3 factors activate genes / ATF6-mediated unfolded protein response / regulation of cholesterol biosynthetic process / ATF6 (ATF6-alpha) activates chaperones / ATF6B (ATF6-beta) activates chaperones / Assembly of active LPL and LIPC lipase complexes / membrane protein intracellular domain proteolysis / regulation of vesicle-mediated transport / Regulation of cholesterol biosynthesis by SREBP (SREBF) / Golgi stack / lysosome organization / mitotic G2 DNA damage checkpoint signaling / cholesterol metabolic process / endoplasmic reticulum unfolded protein response / response to endoplasmic reticulum stress / protein maturation / Post-translational protein phosphorylation / protein processing / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / protein import into nucleus / endoplasmic reticulum lumen / Golgi membrane / serine-type endopeptidase activity / endoplasmic reticulum membrane / Golgi apparatus / proteolysis Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
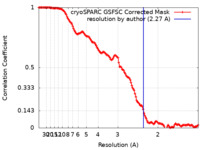
| Method | single particle reconstruction / cryo EM / Resolution: 2.27 Å | |||||||||
 Authors Authors | Kober DL | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: SPRING licenses S1P-mediated cleavage of SREBP2 by displacing an inhibitory pro-domain. Authors: Sebastian Hendrix / Vincent Dartigue / Hailee Hall / Shrankhla Bawaria / Jenina Kingma / Bilkish Bajaj / Noam Zelcer / Daniel L Kober /   Abstract: Site-one protease (S1P) conducts the first of two cleavage events in the Golgi to activate Sterol regulatory element binding proteins (SREBPs) and upregulate lipogenic transcription. S1P is also ...Site-one protease (S1P) conducts the first of two cleavage events in the Golgi to activate Sterol regulatory element binding proteins (SREBPs) and upregulate lipogenic transcription. S1P is also required for a wide array of additional signaling pathways. A zymogen serine protease, S1P matures through autoproteolysis of two pro-domains, with one cleavage event in the endoplasmic reticulum (ER) and the other in the Golgi. We recently identified the SREBP regulating gene, (SPRING), which enhances S1P maturation and is necessary for SREBP signaling. Here, we report the cryo-EM structures of S1P and S1P-SPRING at sub-2.5 Å resolution. SPRING activates S1P by dislodging its inhibitory pro-domain and stabilizing intra-domain contacts. Functionally, SPRING licenses S1P to cleave its cognate substrate, SREBP2. Our findings reveal an activation mechanism for S1P and provide insights into how spatial control of S1P activity underpins cholesterol homeostasis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42661.map.gz emd_42661.map.gz | 123.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42661-v30.xml emd-42661-v30.xml emd-42661.xml emd-42661.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42661_fsc.xml emd_42661_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_42661.png emd_42661.png | 134.4 KB | ||
| Filedesc metadata |  emd-42661.cif.gz emd-42661.cif.gz | 6.5 KB | ||
| Others |  emd_42661_additional_1.map.gz emd_42661_additional_1.map.gz emd_42661_half_map_1.map.gz emd_42661_half_map_1.map.gz emd_42661_half_map_2.map.gz emd_42661_half_map_2.map.gz | 126.3 MB 226.8 MB 226.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42661 http://ftp.pdbj.org/pub/emdb/structures/EMD-42661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42661 | HTTPS FTP |
-Validation report
| Summary document |  emd_42661_validation.pdf.gz emd_42661_validation.pdf.gz | 749.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42661_full_validation.pdf.gz emd_42661_full_validation.pdf.gz | 749.5 KB | Display | |
| Data in XML |  emd_42661_validation.xml.gz emd_42661_validation.xml.gz | 22.3 KB | Display | |
| Data in CIF |  emd_42661_validation.cif.gz emd_42661_validation.cif.gz | 29 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42661 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42661 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42661 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42661 | HTTPS FTP |
-Related structure data
| Related structure data |  8uwcMC  8uw8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42661.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42661.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Primary, unsharpened map for S1P | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.738 Å | ||||||||||||||||||||||||||||||||||||
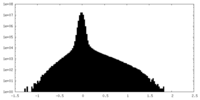
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharpened map (B factor = -62)
| File | emd_42661_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map (B factor = -62) | ||||||||||||
| Projections & Slices |
| ||||||||||||
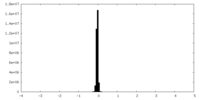
| Density Histograms |
-Half map: half map A
| File | emd_42661_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
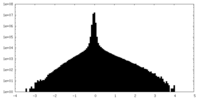
| Density Histograms |
-Half map: half map B
| File | emd_42661_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
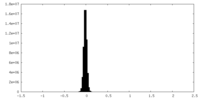
| Density Histograms |
- Sample components
Sample components
-Entire : Matured site-1 protease
| Entire | Name: Matured site-1 protease |
|---|---|
| Components |
|
-Supramolecule #1: Matured site-1 protease
| Supramolecule | Name: Matured site-1 protease / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: FLAG-S1P-His purified using NiNTA chromatography and gel filtration. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 120 KDa |
-Macromolecule #1: Membrane-bound transcription factor site-1 protease
| Macromolecule | Name: Membrane-bound transcription factor site-1 protease / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: site-1 protease |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 113.883789 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: METDTLLLWV LLLWVPGSTG DYKDDDDKDR LEKKSFEKAP CPGCSHLTLK VEFSSTVVEY EYIVAFNGYF TAKARNSFIS SALKSSEVD NWRIIPRNNP SSDYPSDFEV IQIKEKQKAG LLTLEDHPNI KRVTPQRKVF RSLKYAESDP TVPCNETRWS Q KWQSSRPL ...String: METDTLLLWV LLLWVPGSTG DYKDDDDKDR LEKKSFEKAP CPGCSHLTLK VEFSSTVVEY EYIVAFNGYF TAKARNSFIS SALKSSEVD NWRIIPRNNP SSDYPSDFEV IQIKEKQKAG LLTLEDHPNI KRVTPQRKVF RSLKYAESDP TVPCNETRWS Q KWQSSRPL RRASLSLGSG FWHATGRHSS RRLLRAIPRQ VAQTLQADVL WQMGYTGANV RVAVFDTGLS EKHPHFKNVK ER TNWTNER TLDDGLGHGT FVAGVIASMR ECQGFAPDAE LHIFRVFTNN QVSYTSWFLD AFNYAILKKI DVLNLSIGGP DFM DHPFVD KVWELTANNV IMVSAIGNDG PLYGTLNNPA DQMDVIGVGG IDFEDNIARF SSRGMTTWEL PGGYGRMKPD IVTY GAGVR GSGVKGGCRA LSGTSVASPV VAGAVTLLVS TVQKRELVNP ASMKQALIAS ARRLPGVNMF EQGHGKLDLL RAYQI LNSY KPQASLSPSY IDLTECPYMW PYCSQPIYYG GMPTVVNVTI LNGMGVTGRI VDKPDWQPYL PQNGDNIEVA FSYSSV LWP WSGYLAISIS VTKKAASWEG IAQGHVMITV ASPAETESKN GAEQTSTVKL PIKVKIIPTP PRSKRVLWDQ YHNLRYP PG YFPRDNLRMK NDPLDWNGDH IHTNFRDMYQ HLRSMGYFVE VLGAPFTCFD ASQYGTLLMV DSEEEYFPEE IAKLRRDV D NGLSLVIFSD WYNTSVMRKV KFYDENTRQW WMPDTGGANI PALNELLSVW NMGFSDGLYE GEFTLANHDM YYASGCSIA KFPEDGVVIT QTFKDQGLEV LKQETAVVEN VPILGLYQIP AEGGGRIVLY GDSNCLDDSH RQKDCFWLLD ALLQYTSYGV TPPSLSHSG NRQRPPSGAG SVTPERMEGN HLHRYSKVLE AHLGDPKPRP LPACPRLSWA KPQPLNETAP SNLWKHQKLL S IDLDKVVL PNFRSNRPQV RPLSPGESGA WDIPGGIMPG RYNQEVHHHH HHHHHH UniProtKB: Membrane-bound transcription factor site-1 protease |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 97 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.6 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 50 mM HEPES-NaOH and 150 mM NaCl | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris / Energy filter - Slit width: 5 eV |
| Image recording | Film or detector model: TFS FALCON 4i (4k x 4k) / Number grids imaged: 1 / Number real images: 6139 / Average exposure time: 3.5 sec. / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)