+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pendrin in apo | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chloride transport / iodide transport / bicarbonate transport / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationiodide transmembrane transporter activity / secondary active sulfate transmembrane transporter activity / regulation of pH / chloride:bicarbonate antiporter activity / brush border membrane / regulation of protein localization / apical plasma membrane / extracellular exosome Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Wang L / Hoang A / Zhou M | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Mechanism of anion exchange and small-molecule inhibition of pendrin. Authors: Lie Wang / Anthony Hoang / Eva Gil-Iturbe / Arthur Laganowsky / Matthias Quick / Ming Zhou /  Abstract: Pendrin (SLC26A4) is an anion exchanger that mediates bicarbonate (HCO) exchange for chloride (Cl) and is crucial for maintaining pH and salt homeostasis in the kidney, lung, and cochlea. Pendrin ...Pendrin (SLC26A4) is an anion exchanger that mediates bicarbonate (HCO) exchange for chloride (Cl) and is crucial for maintaining pH and salt homeostasis in the kidney, lung, and cochlea. Pendrin also exports iodide (I) in the thyroid gland. Pendrin mutations in humans lead to Pendred syndrome, causing hearing loss and goiter. Inhibition of pendrin is a validated approach for attenuating airway hyperresponsiveness in asthma and for treating hypertension. However, the mechanism of anion exchange and its inhibition by drugs remains poorly understood. We applied cryo-electron microscopy to determine structures of pendrin from Sus scrofa in the presence of either Cl, I, HCO or in the apo-state. The structures reveal two anion-binding sites in each protomer, and functional analyses show both sites are involved in anion exchange. The structures also show interactions between the Sulfate Transporter and Anti-Sigma factor antagonist (STAS) and transmembrane domains, and mutational studies suggest a regulatory role. We also determine the structure of pendrin in a complex with niflumic acid (NFA), which uncovers a mechanism of inhibition by competing with anion binding and impeding the structural changes necessary for anion exchange. These results reveal directions for understanding the mechanisms of anion selectivity and exchange and their regulations by the STAS domain. This work also establishes a foundation for analyzing the pathophysiology of mutations associated with Pendred syndrome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42588.map.gz emd_42588.map.gz | 32.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42588-v30.xml emd-42588-v30.xml emd-42588.xml emd-42588.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42588.png emd_42588.png | 35.9 KB | ||
| Filedesc metadata |  emd-42588.cif.gz emd-42588.cif.gz | 6.7 KB | ||
| Others |  emd_42588_half_map_1.map.gz emd_42588_half_map_1.map.gz emd_42588_half_map_2.map.gz emd_42588_half_map_2.map.gz | 59 MB 59 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42588 http://ftp.pdbj.org/pub/emdb/structures/EMD-42588 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42588 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42588 | HTTPS FTP |
-Related structure data
| Related structure data |  8uukMC  8sgwC  8sh3C  8shcC  8sieC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42588.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42588.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||||||||||||||||||
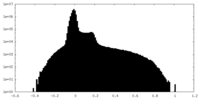
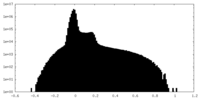
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_42588_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
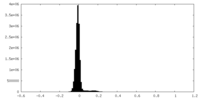
| Density Histograms |
-Half map: #1
| File | emd_42588_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
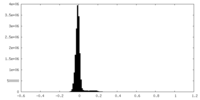
| Density Histograms |
- Sample components
Sample components
-Entire : homodimer of Pendrin
| Entire | Name: homodimer of Pendrin |
|---|---|
| Components |
|
-Supramolecule #1: homodimer of Pendrin
| Supramolecule | Name: homodimer of Pendrin / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 75 KDa |
-Macromolecule #1: Pendrin
| Macromolecule | Name: Pendrin / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 86.113273 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MAAPGSRLEP PPLPEYSCSY VVSRPVYSEL AFQQQYERRL QERKTLRESL AKRCSCSRKR TLGVLKTLLP VLDWLPKYRI KEWLLSDII SGVSTGLVGT LQGMAYALLA AVPVGYGLYS AFFPILTYFI FGTSRHISVG PFPVVSLMVG SVVLSMAPDE H FIISSSNG ...String: MAAPGSRLEP PPLPEYSCSY VVSRPVYSEL AFQQQYERRL QERKTLRESL AKRCSCSRKR TLGVLKTLLP VLDWLPKYRI KEWLLSDII SGVSTGLVGT LQGMAYALLA AVPVGYGLYS AFFPILTYFI FGTSRHISVG PFPVVSLMVG SVVLSMAPDE H FIISSSNG TALNTTVIDF AARDAARVLI ASTLTLLVGI IQLIFGGLQI GFIVRYLADP LVGGFTTAAA FQVLVSQLKI VL NVSTKNY NGILSIIYTL IEIFQNIGNT NLADFIAGLL TIIICMAVKE LNDRFKHKIP VPIPIEVIVT IIATAISYAV NLE KNYNAG IVKSIPRGFL PPEIPPISLF SEMLTASFSI AVVAYAIAVS VGKVYAIKYD YTIDGNQEFI AFGISNIFSG FFSC FVATT ALSRTAVQES TGGKTQIAGI ISAAVVMIAI VALGKLLEPL QKSVLAAVVI ANLKGMFMQV CDVPRLWRQN KTDAV IWVF TCIASIILGL DLGLLAGLMF GFLTVVVRVQ FPSWNSLGSI PNTDIYRSTK DYKNIEEPEG VKILRFSSPI FYGNVD GLK KCIKSTVGFD AIRVYNKRLK ALRKIQKLIK KGQLRATKNG IISDAGSSNN AFEPDEDIED PEELDIPTKE IEIQVDW NS ELPVKVNVPK VPIHSLVLDC GAVSFLDVVG VRSLRMIVKE FQRIDVHVYF ASLQDHVIEK LEQCGFFNDS IRKDIFFL T VHDAILHLRS QVKSQEVQDS ILETITLIQD CKDPLELMEA ELIEEELDVQ DEAMRRLAS UniProtKB: STAS domain-containing protein |
-Macromolecule #2: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 2 / Number of copies: 12 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #3: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
| Macromolecule | Name: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine / type: ligand / ID: 3 / Number of copies: 14 / Formula: LBN |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-LBN: |
-Macromolecule #4: Lauryl Maltose Neopentyl Glycol
| Macromolecule | Name: Lauryl Maltose Neopentyl Glycol / type: ligand / ID: 4 / Number of copies: 4 / Formula: AV0 |
|---|---|
| Molecular weight | Theoretical: 1.005188 KDa |
| Chemical component information |  ChemComp-AV0: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)