[English] 日本語
 Yorodumi
Yorodumi- EMDB-42555: Refined map of the Listeria innocua 70S ribosome (head-swiveled) ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Refined map of the Listeria innocua 70S ribosome (head-swiveled) in complex with pe/E-tRNA (structure I-B) | |||||||||||||||
 Map data Map data | Listeria innocua 70S head-swiveled ribosome bound to pe/E tRNA (Structure I-B) Main Map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | cryo-EM / recycling / time-resolved / RIBOSOME | |||||||||||||||
| Biological species |  Listeria innocua (bacteria) Listeria innocua (bacteria) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||||||||
 Authors Authors | Seely SM / Basu R / Gagnon MG | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2024 Journal: Nucleic Acids Res / Year: 2024Title: Mechanistic insights into the alternative ribosome recycling by HflXr. Authors: Savannah M Seely / Ritwika S Basu / Matthieu G Gagnon /  Abstract: During stress conditions such as heat shock and antibiotic exposure, ribosomes stall on messenger RNAs, leading to inhibition of protein synthesis. To remobilize ribosomes, bacteria use rescue ...During stress conditions such as heat shock and antibiotic exposure, ribosomes stall on messenger RNAs, leading to inhibition of protein synthesis. To remobilize ribosomes, bacteria use rescue factors such as HflXr, a homolog of the conserved housekeeping GTPase HflX that catalyzes the dissociation of translationally inactive ribosomes into individual subunits. Here we use time-resolved cryo-electron microscopy to elucidate the mechanism of ribosome recycling by Listeria monocytogenes HflXr. Within the 70S ribosome, HflXr displaces helix H69 of the 50S subunit and induces long-range movements of the platform domain of the 30S subunit, disrupting inter-subunit bridges B2b, B2c, B4, B7a and B7b. Our findings unveil a unique ribosome recycling strategy by HflXr which is distinct from that mediated by RRF and EF-G. The resemblance between HflXr and housekeeping HflX suggests that the alternative ribosome recycling mechanism reported here is universal in the prokaryotic kingdom. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42555.map.gz emd_42555.map.gz | 254 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42555-v30.xml emd-42555-v30.xml emd-42555.xml emd-42555.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42555.png emd_42555.png | 117.4 KB | ||
| Masks |  emd_42555_msk_1.map emd_42555_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42555.cif.gz emd-42555.cif.gz | 4.6 KB | ||
| Others |  emd_42555_additional_1.map.gz emd_42555_additional_1.map.gz emd_42555_half_map_1.map.gz emd_42555_half_map_1.map.gz emd_42555_half_map_2.map.gz emd_42555_half_map_2.map.gz | 263.7 MB 474.9 MB 474.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42555 http://ftp.pdbj.org/pub/emdb/structures/EMD-42555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42555 | HTTPS FTP |
-Validation report
| Summary document |  emd_42555_validation.pdf.gz emd_42555_validation.pdf.gz | 992 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42555_full_validation.pdf.gz emd_42555_full_validation.pdf.gz | 991.5 KB | Display | |
| Data in XML |  emd_42555_validation.xml.gz emd_42555_validation.xml.gz | 19.1 KB | Display | |
| Data in CIF |  emd_42555_validation.cif.gz emd_42555_validation.cif.gz | 22.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42555 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42555 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42555 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42555 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42555.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42555.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Listeria innocua 70S head-swiveled ribosome bound to pe/E tRNA (Structure I-B) Main Map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
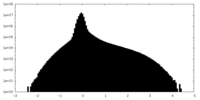
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42555_msk_1.map emd_42555_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
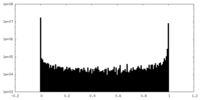
| Density Histograms |
-Additional map: Listeria innocua 70S head-swiveled ribosome bound to pe/E...
| File | emd_42555_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Listeria innocua 70S head-swiveled ribosome bound to pe/E tRNA (Structure I-B) Sharpened Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
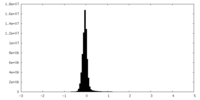
| Density Histograms |
-Half map: Listeria innocua 70S head-swiveled ribosome bound to pe/E...
| File | emd_42555_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Listeria innocua 70S head-swiveled ribosome bound to pe/E tRNA (Structure I-B) Half Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
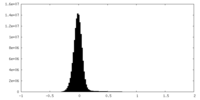
| Density Histograms |
-Half map: Listeria innocua 70S head-swiveled ribosome bound to pe/E...
| File | emd_42555_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Listeria innocua 70S head-swiveled ribosome bound to pe/E tRNA (Structure I-B) Half Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Listeria innocua 70S ribosome in complex with pe/E-tRNA
| Entire | Name: Listeria innocua 70S ribosome in complex with pe/E-tRNA |
|---|---|
| Components |
|
-Supramolecule #1: Listeria innocua 70S ribosome in complex with pe/E-tRNA
| Supramolecule | Name: Listeria innocua 70S ribosome in complex with pe/E-tRNA type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Listeria innocua (bacteria) Listeria innocua (bacteria) |
| Molecular weight | Theoretical: 2.5 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 295 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Number real images: 7572 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)