+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4186 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Glutamine synthetase I from Mycobacterium smegmatis | |||||||||
 Map data Map data | Glutamine synthetase I from Mycobacterium smegmatis | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Mycobacterium smegmatis str. MC2 155 (bacteria) Mycobacterium smegmatis str. MC2 155 (bacteria) | |||||||||
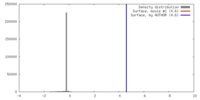
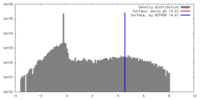
| Method | single particle reconstruction / negative staining / Resolution: 24.0 Å | |||||||||
 Authors Authors | Kirykowicz AM / Woodward JD | |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Glutamine synthetase I from Mycobacterium smegmatis Authors: Kirykowicz AM / Woodward JD | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4186.map.gz emd_4186.map.gz | 145.2 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4186-v30.xml emd-4186-v30.xml emd-4186.xml emd-4186.xml | 8.6 KB 8.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4186.png emd_4186.png | 27 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4186 http://ftp.pdbj.org/pub/emdb/structures/EMD-4186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4186 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_4186.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4186.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Glutamine synthetase I from Mycobacterium smegmatis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Glutamine Synthetase I
| Entire | Name: Glutamine Synthetase I |
|---|---|
| Components |
|
-Supramolecule #1: Glutamine Synthetase I
| Supramolecule | Name: Glutamine Synthetase I / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Mycobacterium smegmatis str. MC2 155 (bacteria) Mycobacterium smegmatis str. MC2 155 (bacteria) |
| Molecular weight | Experimental: 648 KDa |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 50 mM Tris-HCl, 200 mM NaCl |
| Staining | Type: NEGATIVE / Material: Uranyl Acetate / Details: Sample washed/stained with 2% UA and air-dried |
| Grid | Material: COPPER / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Details | Obtained from partially purified fraction |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Image recording | Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 152 / Average exposure time: 0.5 sec. / Average electron dose: 20.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 1.2 mm / Nominal magnification: 50000 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)