登録情報 データベース : EMDB / ID : EMD-41816タイトル Cryo-EM structure of the RAF1-HSP90-CDC37 complex in the closed state Sharpened Map 複合体 : Complex of RAF1-HSP90-CDC37複合体 : Heat shock protein 90タンパク質・ペプチド : Heat shock protein 83複合体 : RAF1 & HSP90 co-chaperone CDC37タンパク質・ペプチド : RAF proto-oncogene serine/threonine-protein kinaseタンパク質・ペプチド : Hsp90 co-chaperone Cdc37, N-terminally processedリガンド : ADENOSINE-5'-TRIPHOSPHATEリガンド : MAGNESIUM ION / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
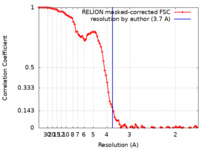
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Trichoplusia ni (イラクサキンウワバ) / Homo sapiens (ヒト)手法 / / 解像度 : 3.7 Å Finci LI / Simanshu DK 資金援助 Organization Grant number 国 National Institutes of Health/National Cancer Institute (NIH/NCI) HHSN261200800001E
ジャーナル : Commun Biol / 年 : 2024タイトル : Structural dynamics of RAF1-HSP90-CDC37 and HSP90 complexes reveal asymmetric client interactions and key structural elements.著者 : Lorenzo I Finci / Mayukh Chakrabarti / Gulcin Gulten / Joseph Finney / Carissa Grose / Tara Fox / Renbin Yang / Dwight V Nissley / Frank McCormick / Dominic Esposito / Trent E Balius / Dhirendra K Simanshu / 要旨 : RAF kinases are integral to the RAS-MAPK signaling pathway, and proper RAF1 folding relies on its interaction with the chaperone HSP90 and the cochaperone CDC37. Understanding the intricate molecular ... RAF kinases are integral to the RAS-MAPK signaling pathway, and proper RAF1 folding relies on its interaction with the chaperone HSP90 and the cochaperone CDC37. Understanding the intricate molecular interactions governing RAF1 folding is crucial for comprehending this process. Here, we present a cryo-EM structure of the closed-state RAF1-HSP90-CDC37 complex, where the C-lobe of the RAF1 kinase domain binds to one side of the HSP90 dimer, and an unfolded N-lobe segment of the RAF1 kinase domain threads through the center of the HSP90 dimer. CDC37 binds to the kinase C-lobe, mimicking the N-lobe with its HxNI motif. We also describe structures of HSP90 dimers without RAF1 and CDC37, displaying only N-terminal and middle domains, which we term the semi-open state. Employing 1 μs atomistic simulations, energetic decomposition, and comparative structural analysis, we elucidate the dynamics and interactions within these complexes. Our quantitative analysis reveals that CDC37 bridges the HSP90-RAF1 interaction, RAF1 binds HSP90 asymmetrically, and that HSP90 structural elements engage RAF1's unfolded region. Additionally, N- and C-terminal interactions stabilize HSP90 dimers, and molecular interactions in HSP90 dimers rearrange between the closed and semi-open states. Our findings provide valuable insight into the contributions of HSP90 and CDC37 in mediating client folding. 履歴 登録 2023年9月1日 - ヘッダ(付随情報) 公開 2024年3月13日 - マップ公開 2024年3月13日 - 更新 2025年5月14日 - 現状 2025年5月14日 処理サイト : RCSB / 状態 : 公開
すべて表示 表示を減らす
 万見
万見 データを開く
データを開く 基本情報
基本情報
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Trichoplusia ni (イラクサキンウワバ) /
Trichoplusia ni (イラクサキンウワバ) /  Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 米国, 1件
米国, 1件  引用
引用 ジャーナル: Commun Biol / 年: 2024
ジャーナル: Commun Biol / 年: 2024
 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_41816.map.gz
emd_41816.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-41816-v30.xml
emd-41816-v30.xml emd-41816.xml
emd-41816.xml EMDBヘッダ
EMDBヘッダ emd_41816_fsc.xml
emd_41816_fsc.xml FSCデータファイル
FSCデータファイル emd_41816.png
emd_41816.png emd-41816.cif.gz
emd-41816.cif.gz emd_41816_additional_1.map.gz
emd_41816_additional_1.map.gz emd_41816_half_map_1.map.gz
emd_41816_half_map_1.map.gz emd_41816_half_map_2.map.gz
emd_41816_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-41816
http://ftp.pdbj.org/pub/emdb/structures/EMD-41816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41816
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41816 emd_41816_validation.pdf.gz
emd_41816_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_41816_full_validation.pdf.gz
emd_41816_full_validation.pdf.gz emd_41816_validation.xml.gz
emd_41816_validation.xml.gz emd_41816_validation.cif.gz
emd_41816_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41816
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41816 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41816
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41816 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_41816.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_41816.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ) Homo sapiens (ヒト)
Homo sapiens (ヒト) Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ) Homo sapiens (ヒト)
Homo sapiens (ヒト) Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ) Homo sapiens (ヒト)
Homo sapiens (ヒト) Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ)
 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 ムービー
ムービー コントローラー
コントローラー


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)