[English] 日本語
 Yorodumi
Yorodumi- EMDB-41528: Polyclonal immune complex of Fab binding the H2 HA from serum of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Polyclonal immune complex of Fab binding the H2 HA from serum of subject 2-2 week 16 | |||||||||
 Map data Map data | Negative stain map of global refinement of particle stack used to sort polyclonal responses to H2 HA from human subject 2-2 at week 16 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | influenza / hemagglutinin / H2 / polyclonal complex / Fab complex / viral fusion protein / VIRAL PROTEIN | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 20.0 Å | |||||||||
 Authors Authors | Yang YR / Han J / Richey ST / Ward AB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2024 Journal: Cell Rep / Year: 2024Title: Immune memory shapes human polyclonal antibody responses to H2N2 vaccination. Authors: Yuhe R Yang / Julianna Han / Hailee R Perrett / Sara T Richey / Alesandra J Rodriguez / Abigail M Jackson / Rebecca A Gillespie / Sarah O'Connell / Julie E Raab / Lauren Y Cominsky / Ankita ...Authors: Yuhe R Yang / Julianna Han / Hailee R Perrett / Sara T Richey / Alesandra J Rodriguez / Abigail M Jackson / Rebecca A Gillespie / Sarah O'Connell / Julie E Raab / Lauren Y Cominsky / Ankita Chopde / Masaru Kanekiyo / Katherine V Houser / Grace L Chen / Adrian B McDermott / Sarah F Andrews / Andrew B Ward /   Abstract: Influenza A virus subtype H2N2, which caused the 1957 influenza pandemic, remains a global threat. A recent phase 1 clinical trial investigating a ferritin nanoparticle vaccine displaying H2 ...Influenza A virus subtype H2N2, which caused the 1957 influenza pandemic, remains a global threat. A recent phase 1 clinical trial investigating a ferritin nanoparticle vaccine displaying H2 hemagglutinin (HA) in H2-naive and H2-exposed adults enabled us to perform comprehensive structural and biochemical characterization of immune memory on the breadth and diversity of the polyclonal serum antibody response elicited. We temporally map the epitopes targeted by serum antibodies after vaccine prime and boost, revealing that previous H2 exposure results in higher responses to the variable HA head domain. In contrast, initial responses in H2-naive participants are dominated by antibodies targeting conserved epitopes. We use cryoelectron microscopy and monoclonal B cell isolation to describe the molecular details of cross-reactive antibodies targeting conserved epitopes on the HA head, including the receptor-binding site and a new site of vulnerability deemed the medial junction. Our findings accentuate the impact of pre-existing influenza exposure on serum antibody responses post-vaccination. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41528.map.gz emd_41528.map.gz | 11.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41528-v30.xml emd-41528-v30.xml emd-41528.xml emd-41528.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41528.png emd_41528.png | 44 KB | ||
| Filedesc metadata |  emd-41528.cif.gz emd-41528.cif.gz | 4.6 KB | ||
| Others |  emd_41528_additional_1.map.gz emd_41528_additional_1.map.gz emd_41528_half_map_1.map.gz emd_41528_half_map_1.map.gz emd_41528_half_map_2.map.gz emd_41528_half_map_2.map.gz | 11.9 MB 11.9 MB 11.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41528 http://ftp.pdbj.org/pub/emdb/structures/EMD-41528 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41528 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41528 | HTTPS FTP |
-Validation report
| Summary document |  emd_41528_validation.pdf.gz emd_41528_validation.pdf.gz | 568.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41528_full_validation.pdf.gz emd_41528_full_validation.pdf.gz | 567.8 KB | Display | |
| Data in XML |  emd_41528_validation.xml.gz emd_41528_validation.xml.gz | 9.6 KB | Display | |
| Data in CIF |  emd_41528_validation.cif.gz emd_41528_validation.cif.gz | 11.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41528 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41528 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41528 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41528 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41528.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41528.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain map of global refinement of particle stack used to sort polyclonal responses to H2 HA from human subject 2-2 at week 16 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.06 Å | ||||||||||||||||||||||||||||||||||||
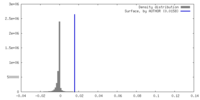
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Negative stain map of polyclonal Fab binding the...
| File | emd_41528_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain map of polyclonal Fab binding the H2 HA central stem epitope from human subject 2-2 at week 16 | ||||||||||||
| Projections & Slices |
| ||||||||||||
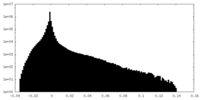
| Density Histograms |
-Half map: Negative stain map of half map A for...
| File | emd_41528_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain map of half map A for global refinement of particle stack used to sort polyclonal responses to H2 HA from human subject 2-2 at week 16 | ||||||||||||
| Projections & Slices |
| ||||||||||||
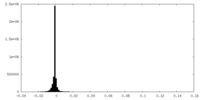
| Density Histograms |
-Half map: Negative stain map of half map B for...
| File | emd_41528_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain map of half map B for global refinement of particle stack used to sort polyclonal responses to H2 HA from human subject 2-2 at week 16 | ||||||||||||
| Projections & Slices |
| ||||||||||||
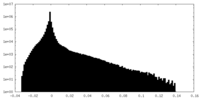
| Density Histograms |
- Sample components
Sample components
-Entire : Polyclonal immune complex of Fab binding the H2 HA from serum of ...
| Entire | Name: Polyclonal immune complex of Fab binding the H2 HA from serum of subject 2-2 at week 16 |
|---|---|
| Components |
|
-Supramolecule #1: Polyclonal immune complex of Fab binding the H2 HA from serum of ...
| Supramolecule | Name: Polyclonal immune complex of Fab binding the H2 HA from serum of subject 2-2 at week 16 type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 280 KDa |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.015 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Staining | Type: NEGATIVE / Material: 2% w/v uranyl formate |
| Grid | Model: Homemade / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: FEI EAGLE (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Nominal defocus max: -2.0 µm / Nominal defocus min: -2.0 µm |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















































































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)