[English] 日本語
 Yorodumi
Yorodumi- EMDB-41253: Cryo-EM map of the Saccharomyces cerevisiae clamp unloader Elg1-R... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
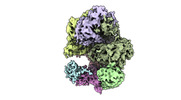
| Title | Cryo-EM map of the Saccharomyces cerevisiae clamp unloader Elg1-RFC bound to a cracked PCNA | ||||||||||||
 Map data Map data | Cryo-EM density map of Elg1-RFC complexed with cracked PCNA clamp | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | DNA replication / DNA sliding clamp / PCNA clamp / clamp loader/unloader / Elg1-RFC unloader / REPLICATION | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationRad17 RFC-like complex / : / Elg1 RFC-like complex / DNA replication factor C complex / Ctf18 RFC-like complex / Polymerase switching / Translesion synthesis by REV1 / : / : / : ...Rad17 RFC-like complex / : / Elg1 RFC-like complex / DNA replication factor C complex / Ctf18 RFC-like complex / Polymerase switching / Translesion synthesis by REV1 / : / : / : / DNA replication checkpoint signaling / PCNA complex / : / Activation of ATR in response to replication stress / lagging strand elongation / sister chromatid cohesion / mitotic sister chromatid cohesion / error-free translesion synthesis / DNA polymerase processivity factor activity / leading strand elongation / Gap-filling DNA repair synthesis and ligation in TC-NER / regulation of DNA replication / Dual incision in TC-NER / mismatch repair / DNA damage checkpoint signaling / DNA-templated DNA replication / DNA repair / ATP hydrolysis activity / DNA binding / ATP binding / nucleus / cytosol Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.67 Å | ||||||||||||
 Authors Authors | Zheng F / Yao YN / Georgescu R / O'Donnell ME / Li H | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2024 Journal: Sci Adv / Year: 2024Title: Structure of the PCNA unloader Elg1-RFC. Authors: Fengwei Zheng / Nina Y Yao / Roxana E Georgescu / Huilin Li / Michael E O'Donnell /  Abstract: During DNA replication, the proliferating cell nuclear antigen (PCNA) clamps are loaded onto primed sites for each Okazaki fragment synthesis by the AAA heteropentamer replication factor C (RFC). ...During DNA replication, the proliferating cell nuclear antigen (PCNA) clamps are loaded onto primed sites for each Okazaki fragment synthesis by the AAA heteropentamer replication factor C (RFC). PCNA encircling duplex DNA is quite stable and is removed from DNA by the dedicated clamp unloader Elg1-RFC. Here, we show the cryo-EM structure of Elg1-RFC in various states with PCNA. The structures reveal essential features of Elg1-RFC that explain how it is dedicated to PCNA unloading. Specifically, Elg1 contains two external loops that block opening of the Elg1-RFC complex for DNA binding, and an "Elg1 plug" domain that fills the central DNA binding chamber, thereby reinforcing the exclusive PCNA unloading activity of Elg1-RFC. Elg1-RFC was capable of unloading PCNA using non-hydrolyzable AMP-PNP. Both RFC and Elg1-RFC could remove PCNA from covalently closed circular DNA, indicating that PCNA unloading occurs by a mechanism that is distinct from PCNA loading. Implications for the PCNA unloading mechanism are discussed. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41253.map.gz emd_41253.map.gz | 267 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41253-v30.xml emd-41253-v30.xml emd-41253.xml emd-41253.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41253.png emd_41253.png | 57.9 KB | ||
| Filedesc metadata |  emd-41253.cif.gz emd-41253.cif.gz | 8 KB | ||
| Others |  emd_41253_half_map_1.map.gz emd_41253_half_map_1.map.gz emd_41253_half_map_2.map.gz emd_41253_half_map_2.map.gz | 262.4 MB 262.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41253 http://ftp.pdbj.org/pub/emdb/structures/EMD-41253 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41253 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41253 | HTTPS FTP |
-Validation report
| Summary document |  emd_41253_validation.pdf.gz emd_41253_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41253_full_validation.pdf.gz emd_41253_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_41253_validation.xml.gz emd_41253_validation.xml.gz | 16.6 KB | Display | |
| Data in CIF |  emd_41253_validation.cif.gz emd_41253_validation.cif.gz | 19.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41253 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41253 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41253 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41253 | HTTPS FTP |
-Related structure data
| Related structure data |  8thcMC  8thbC  8thdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41253.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41253.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density map of Elg1-RFC complexed with cracked PCNA clamp | ||||||||||||||||||||||||||||||||||||
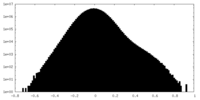
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
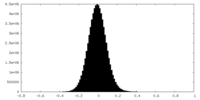
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM density map of Elg1-RFC complexed with cracked...
| File | emd_41253_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density map of Elg1-RFC complexed with cracked PCNA clamp_half-A | ||||||||||||
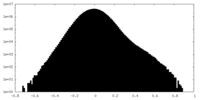
| Projections & Slices |
| ||||||||||||
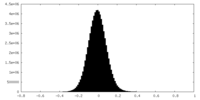
| Density Histograms |
-Half map: Cryo-EM density map of Elg1-RFC complexed with cracked...
| File | emd_41253_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density map of Elg1-RFC complexed with cracked PCNA clamp_half-B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : PCNA clamp unloader Elg1-RFC
+Supramolecule #1: PCNA clamp unloader Elg1-RFC
+Supramolecule #2: PCNA clamp unloader Elg1-RFC
+Supramolecule #3: PCNA clamp
+Macromolecule #1: ELG1 isoform 1
+Macromolecule #2: Replication factor C subunit 4
+Macromolecule #3: Replication factor C subunit 3
+Macromolecule #4: Replication factor C subunit 2
+Macromolecule #5: Replication factor C subunit 5
+Macromolecule #6: Proliferating cell nuclear antigen
+Macromolecule #7: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
+Macromolecule #8: MAGNESIUM ION
+Macromolecule #9: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.1 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)