[English] 日本語
 Yorodumi
Yorodumi- EMDB-40195: The Type 9 Secretion System in vitro assembled, FspA-CTD substrat... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The Type 9 Secretion System in vitro assembled, FspA-CTD substrate bound complex | |||||||||||||||||||||
 Map data Map data | refined volume | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | T9SS / Type IX Secretion System Translocon / Protein Secretion PorV - SprA - PPI - FspA Complex / MEMBRANE PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpeptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / serine-type endopeptidase activity / proteolysis Similarity search - Function | |||||||||||||||||||||
| Biological species |  Flavobacterium johnsoniae UW101 (bacteria) / Flavobacterium johnsoniae UW101 (bacteria) /  Flavobacterium johnsoniae (bacteria) Flavobacterium johnsoniae (bacteria) | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||||||||||||||
 Authors Authors | Deme JC / Lea SM | |||||||||||||||||||||
| Funding support |  United Kingdom, European Union, United Kingdom, European Union,  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2024 Journal: Nat Microbiol / Year: 2024Title: The Type 9 Secretion System caught in the act of transport Authors: Lauber F / Deme JC / Liu X / Kjaer A / Miller HL / Alcock F / Lea SM / Berks BC | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40195.map.gz emd_40195.map.gz | 80.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40195-v30.xml emd-40195-v30.xml emd-40195.xml emd-40195.xml | 26.1 KB 26.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40195_fsc.xml emd_40195_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_40195.png emd_40195.png | 55.5 KB | ||
| Masks |  emd_40195_msk_1.map emd_40195_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-40195.cif.gz emd-40195.cif.gz | 8.1 KB | ||
| Others |  emd_40195_additional_1.map.gz emd_40195_additional_1.map.gz emd_40195_half_map_1.map.gz emd_40195_half_map_1.map.gz emd_40195_half_map_2.map.gz emd_40195_half_map_2.map.gz | 65.2 MB 80.9 MB 80.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40195 http://ftp.pdbj.org/pub/emdb/structures/EMD-40195 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40195 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40195 | HTTPS FTP |
-Related structure data
| Related structure data |  8gljMC  8gl6C  8gl8C  8glkC  8glmC  8glnC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40195.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40195.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | refined volume | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||
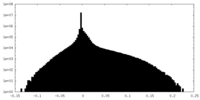
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40195_msk_1.map emd_40195_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
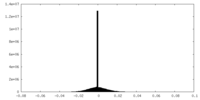
| Density Histograms |
-Additional map: local resolution filtered
| File | emd_40195_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | local resolution filtered | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_40195_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1
| File | emd_40195_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Type 9 Secretion System Extended Translocon
| Entire | Name: Type 9 Secretion System Extended Translocon |
|---|---|
| Components |
|
-Supramolecule #1: Type 9 Secretion System Extended Translocon
| Supramolecule | Name: Type 9 Secretion System Extended Translocon / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2, #4 |
|---|---|
| Source (natural) | Organism:  Flavobacterium johnsoniae UW101 (bacteria) Flavobacterium johnsoniae UW101 (bacteria) |
-Macromolecule #1: Protein involved in gliding motility SprA
| Macromolecule | Name: Protein involved in gliding motility SprA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Flavobacterium johnsoniae (bacteria) Flavobacterium johnsoniae (bacteria) |
| Molecular weight | Theoretical: 270.221688 KDa |
| Sequence | String: MRKICIFLLV LFCGNVLRSQ VKPAVQDTTK TQFSVGKMEL ENPPSILSAY KYDPITDRYI YTTSVDGFSI DYPLVLTPKE YEDLLLKES RRDYFRKKMD AIDGKKTGAE AAKKDLLPRY YINSSLFESI FGSNTIDVKP TGSVEMDLGV RYTKQDNPAF S PRNRSSLT ...String: MRKICIFLLV LFCGNVLRSQ VKPAVQDTTK TQFSVGKMEL ENPPSILSAY KYDPITDRYI YTTSVDGFSI DYPLVLTPKE YEDLLLKES RRDYFRKKMD AIDGKKTGAE AAKKDLLPRY YINSSLFESI FGSNTIDVKP TGSVEMDLGV RYTKQDNPAF S PRNRSSLT FDFDQRISMS LMGKIGTRLE VNANYDTQST FAFQNLFKLA YTPSEDDIIQ KVEVGNVSMP LNSTLIRGAQ SL FGVKTQL QFGRTTITGV FSEQKSQTKS VVAENGGTVQ NFDLYALDYD NDRHFFLSQY FRNKYDVSLK NYPFIDSRVQ ITR LEVWVT NKQNRVTTTG GGNNLRNIIA LQDLGEAQVS GVPDNEVVVI SSTAGFFNNP IDSPTSNTNN KYDPATIGQA GSFL NSNIR EIVTAKSGFN NTNVSEATDY SVLENARKLT TNEYTFNPQL GYISLQQRLA NDEILAVAFE YTVGGKVYQV GEFGS DGVD ATVVTGNNSS NQAIITQSLV LKMLKSNLTN VKNPVWNLMM KNVYQIPQAY QIKQDDFRLN ILYTDPSPIN YITPVQ GSS FPPNPAPDSK VEQTPLLNVF NLDRLNYNND PQAGGDGFFD YIPGVTVDVQ NGRVIFTTKE PFGELIFNKL QTGAGES YN DPTTYNANQQ KYVFRNMYRN TQAGALQDSD KNKFLLRGKY KSSGSNGIPI GAFNVPQGSV VVTAAGRVLV EGIDYSVD Y QLGRVQILDP SLQASNTPIE VSLENNSIFG QQTRRFMGFN IEHKISDKFV IGGTYLKMTE RPFTQKSTYG QESVNNTIF GFNGNYSTEV PFLTRLANKL PNIDTDVPSN LSIRGEVAFL RPDAPKASDF QGEATIYVDD FEGSQSTIDM RSAYAWSLAS TPFITSIND NTFNANSNTL EYGFKRAKLS WYTIDPVFYS SKPSGISNDD LSLNTTRRIY SRELYPNTDI AQGQIQVVNT L DLTYYPGE RGPYNNNPSF GASNPSANFG GIMRALNSTN FEQGNVEYIQ FWVLDPYVGN GESPATNAGK IYFNLGEISE DV LKDGRKQ YENGLGPDQV MVNPQPLWGD VPASQSLIYA FDTNPDNRKN QDVGLDGLPS SREGSIYTNY AGEADPAGDD YTY YLNADG GVLERYKNYN GTEGNSAVSI NDPNRGSTTL PDVEDINRDN TMSTINAYYE YSIDVKPGMQ VGENYITDIR EVTN VDLPN GGTTNARWIQ FKIPVSQPQN TIGNITDFRS IRFMRMFMTG FNSQMTVRFG ALDLVRGEWR RYTGTLDAND QNPDD DGVE FDVAAVNIQE NGTKCPVNYV MPPGVQREQL YNNNTVINQN EQALAVRIGG AGLQYQDSRA VFKNVSVDMR QYKKLK MFL HAESLPNQPT LEDDEMVGFI RFGNDFTQNF YQVEIPLKVT KTGGSCSISP DLVWMDDNSI DLALDLLTRM KIKAMSI DI NSSKRDVNGI YYPDNDPDLE GGDGDGKLTL GIKGNPNFGL VRNLMVGVKS RADHKDIKGE VWFNELRLAD LENKGGMA A ILNVDTNMAD FATVSATGRK STIGFGSLEQ GANERDREDV QQYNIVTNLN LGKLLPKKWG INLPFNYAIG EEVITPEYD PFNQDIKLDQ LIRETTDQAE KDNIRTRAID YTKRKSINFI GVRKDRAPEQ KPHVYDIENF TFSQSYNQVE RHDYEVADYE DEQSNSAVN YAYTFQPKEV VPFKSTKFMK KSEYWKLLSD FNFNYLPSNI SFNTNILRQS NRQQFREVEV EGIGLDPLYR R NFAFNYQY GFGFNLTKSL KLNYSATSNN IVRNFLNDDN SPKEDFNIWD DYLDIGTPNQ HAQQLVLNYD IPINKIPIFG FV KASYSYT ADYMWQRSST AFSEYEDPNG TVYDLGNTIQ NSNSNTLTTT LNMNTLYKYL GLTPGAKKTA KPKTAAPPKP GEK IVNTAK PVVSSSPFYD GLIGVLTSIK NVQINYTKNS GTVLPGYTPS VGFLGTSKPS LGFVFGSQDD VRYEAAKRGW LTTY QDFNQ SFTQVSNKLL KVTANIDLLP DLKVDLSMDR SYSENTSEQY SVDPSTNEYK PLSPYTYGMF SISTVMIKTA FSPSD ETQS AAFDDFRSNR LIIANRLAEG HYGSGVAIPR YGDANNPIPA ETDPNYAVYT ANQGYPIGYT KSNQAVLLPA FLAAYT GSD ASSSSTNIFR SFPIPNWSIK YNGLMRYKYF KDKFKRFSLQ HNYRASYTIN QFRSNFDYNS SPKVQDVNTN FYNEIIM SN VNLVEQFSPL IRMDFELKSS LRVLSEIKKD RALSMSFDNN LLTEVKGMEY IIGLGYRFKD VIFSSRLADN PTGIIKSD I NIKADFSLRN NETLVRYLDY DNNQLAAGQN IWSLKLTADY SFSKNLTAIF YYDHSFSKAV ISTSFPLTNI RSGFTLRYN FGN UniProtKB: Protein involved in gliding motility SprA |
-Macromolecule #2: Peptidyl-prolyl cis-trans isomerase
| Macromolecule | Name: Peptidyl-prolyl cis-trans isomerase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Flavobacterium johnsoniae (bacteria) Flavobacterium johnsoniae (bacteria) |
| Molecular weight | Theoretical: 19.219141 KDa |
| Sequence | String: MKQLLTALLS LTLFISCSKD KDEVKDYTAE NEKEIVDYLA QNNLTAQRTN SGLYYIITKE GSSESEGENP GEEENTGEGE NTEENENDG HPTLNSNITV IYKGYFTNGK VFDESTEGVS YSLRTLIPGW KEGIPLLKSG GEIQLFVPAH LGYGSNGNKT V PGGAVLIF EITLVSVN UniProtKB: Peptidyl-prolyl cis-trans isomerase |
-Macromolecule #3: Type IX secretion system protein PorV domain-containing protein
| Macromolecule | Name: Type IX secretion system protein PorV domain-containing protein type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Flavobacterium johnsoniae (bacteria) Flavobacterium johnsoniae (bacteria) |
| Molecular weight | Theoretical: 44.210043 KDa |
| Sequence | String: MKKISLLLIC LLITTFAKAQ DIERPITTGV PFLLVAADAR AAGLGDQGVA TSSDVFSQQW NPAKYAFAED AQGLSISYTP YLTDLANDI SLGQVTYYNK INDRSAFAGS FRYFGFGGIE LRQTGDPNEP TREVNPNEFA LDGSYSLKLS ETFSMAVAAR Y IRSNLKVA ...String: MKKISLLLIC LLITTFAKAQ DIERPITTGV PFLLVAADAR AAGLGDQGVA TSSDVFSQQW NPAKYAFAED AQGLSISYTP YLTDLANDI SLGQVTYYNK INDRSAFAGS FRYFGFGGIE LRQTGDPNEP TREVNPNEFA LDGSYSLKLS ETFSMAVAAR Y IRSNLKVA TEEIDASAAG SFAVDVAGFY QSEEIAYSDF NGRWRAGFNI QNLGPKISYD HDDLSANFLP ANLRVGGGFD FI FDDYNKL GVSLELTKLL VPTPPGPGTP YDANGDGDFT DPGDISQSQA DEANYKKYKD IGWVSGIFKS FGDAPGGFSE ELK EITYSA AAEYMYQDAF AMRLGYYHES PMKGAKQFFS LGAGFKYSMI KVDVSYLFSA SKVKNPLENT LRFSLTFNFG DKYE TY UniProtKB: Type IX secretion system protein PorV domain-containing protein |
-Macromolecule #4: Por secretion system C-terminal sorting domain-containing protein
| Macromolecule | Name: Por secretion system C-terminal sorting domain-containing protein type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Flavobacterium johnsoniae (bacteria) Flavobacterium johnsoniae (bacteria) |
| Molecular weight | Theoretical: 8.365436 KDa |
| Sequence | String: SDFLVYPNPT KSNISFLFDN ETASVSIYSL LGQKLIEKQI TNQNPVLSVE GLTNGLYFYT FDAGSLHKTG KIIKQ UniProtKB: Por secretion system C-terminal sorting domain-containing protein |
-Macromolecule #5: Lauryl Maltose Neopentyl Glycol
| Macromolecule | Name: Lauryl Maltose Neopentyl Glycol / type: ligand / ID: 5 / Number of copies: 1 / Formula: LMN |
|---|---|
| Molecular weight | Theoretical: 1.005188 KDa |
| Chemical component information |  ChemComp-AV0: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)