+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
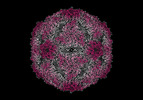
| Title | Cryo-EM structure of enterovirus A71 empty particle | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cryo-EM STRUCTURE / VIRUS / ENTEROVIRUS A71 | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Enterovirus A71 Enterovirus A71 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.96 Å | |||||||||
 Authors Authors | Jiang Y / Huang Y / Zhu R / Zheng Q / Li S / Xia N | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2024 Journal: Nat Microbiol / Year: 2024Title: Broadly therapeutic antibody provides cross-serotype protection against enteroviruses via Fc effector functions and by mimicking SCARB2. Authors: Rui Zhu / Yuanyuan Wu / Yang Huang / Yanan Jiang / Yichao Jiang / Dongqing Zhang / Hui Sun / Zhenhong Zhou / Lizhi Zhou / Shihan Weng / Hao Chen / Xiaoqing Chen / Wenjing Ning / Yuxiang Zou ...Authors: Rui Zhu / Yuanyuan Wu / Yang Huang / Yanan Jiang / Yichao Jiang / Dongqing Zhang / Hui Sun / Zhenhong Zhou / Lizhi Zhou / Shihan Weng / Hao Chen / Xiaoqing Chen / Wenjing Ning / Yuxiang Zou / Maozhou He / Hongwei Yang / Weixi Deng / Yu Li / Zhenqin Chen / Xiangzhong Ye / Jinle Han / Zhichao Yin / Huan Zhao / Che Liu / Yuqiong Que / Mujin Fang / Hai Yu / Jun Zhang / Wenxin Luo / Shaowei Li / Qingbing Zheng / Longfa Xu / Ningshao Xia / Tong Cheng /  Abstract: Enteroviruses contain multiple serotypes and can cause severe neurological complications. The intricate life cycle of enteroviruses involving dynamic virus-receptor interaction hampers the ...Enteroviruses contain multiple serotypes and can cause severe neurological complications. The intricate life cycle of enteroviruses involving dynamic virus-receptor interaction hampers the development of broad therapeutics and vaccines. Here, using function-based screening, we identify a broadly therapeutic antibody h1A6.2 that potently protects mice in lethal models of infection with both enterovirus A71 and coxsackievirus A16 through multiple mechanisms, including inhibition of the virion-SCARB2 interactions and monocyte/macrophage-dependent Fc effector functions. h1A6.2 mitigates inflammation and improves intramuscular mechanics, which are associated with diminished innate immune signalling and preserved tissue repair. Moreover, cryogenic electron microscopy structures delineate an adaptive binding of h1A6.2 to the flexible and dynamic nature of the VP2 EF loop with a binding angle mimicking the SCARB2 receptor. The coordinated binding mode results in efficient binding of h1A6.2 to all viral particle types and facilitates broad neutralization of enterovirus, therefore informing a promising target for the structure-guided design of pan-enterovirus vaccine. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39570.map.gz emd_39570.map.gz | 324.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39570-v30.xml emd-39570-v30.xml emd-39570.xml emd-39570.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_39570.png emd_39570.png | 116.3 KB | ||
| Filedesc metadata |  emd-39570.cif.gz emd-39570.cif.gz | 6.4 KB | ||
| Others |  emd_39570_half_map_1.map.gz emd_39570_half_map_1.map.gz emd_39570_half_map_2.map.gz emd_39570_half_map_2.map.gz | 318.5 MB 318.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39570 http://ftp.pdbj.org/pub/emdb/structures/EMD-39570 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39570 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39570 | HTTPS FTP |
-Validation report
| Summary document |  emd_39570_validation.pdf.gz emd_39570_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39570_full_validation.pdf.gz emd_39570_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_39570_validation.xml.gz emd_39570_validation.xml.gz | 16.5 KB | Display | |
| Data in CIF |  emd_39570_validation.cif.gz emd_39570_validation.cif.gz | 19.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39570 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39570 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39570 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39570 | HTTPS FTP |
-Related structure data
| Related structure data |  8ytbMC  8x95C  8x96C  8x97C  8x98C  8x99C  8x9aC  8x9bC  8ytjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39570.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39570.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
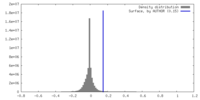
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_39570_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
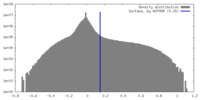
| Density Histograms |
-Half map: #1
| File | emd_39570_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Enterovirus A71
| Entire | Name:   Enterovirus A71 Enterovirus A71 |
|---|---|
| Components |
|
-Supramolecule #1: Enterovirus A71
| Supramolecule | Name: Enterovirus A71 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 39054 / Sci species name: Enterovirus A71 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Enterovirus A71 Enterovirus A71 |
| Molecular weight | Theoretical: 32.730848 KDa |
| Sequence | String: GDRVADVIES SIGDSVSRAL THALPAPTGQ DTQVSSHRLD TGKVPALQAA EIGASSNASD ESMIETRCVL NSHSTAETTL DSFFSRAGL VGEIDLPLKG TTNPNGYANW DIDITGYAQM RRKVELFTYM RFDAEFTFVA CTPTGEVVPQ LLQYMFVPPG A PKPDSRES ...String: GDRVADVIES SIGDSVSRAL THALPAPTGQ DTQVSSHRLD TGKVPALQAA EIGASSNASD ESMIETRCVL NSHSTAETTL DSFFSRAGL VGEIDLPLKG TTNPNGYANW DIDITGYAQM RRKVELFTYM RFDAEFTFVA CTPTGEVVPQ LLQYMFVPPG A PKPDSRES LAWQTATNPS VFVKLSDPPA QVSVPFMSPA SAYQWFYDGY PTFGEHKQEK DLEYGACPNN MMGTFSVRTV GT SKSKYPL VVRIYMRMKH VRAWIPRPMR NQNYLFKANP NYAGNSIKPT GTSRTAITTL UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Enterovirus A71 Enterovirus A71 |
| Molecular weight | Theoretical: 27.726135 KDa |
| Sequence | String: SPSAEACGYS DRVAQLTIGN STITTQEAAN IIVGYGEWPS YCSDSDATAV DKPTRPDVSV NRFYTLDTKL WEKSSKGWYW KFPDVLTET GVFGQNAQFH YLYRSGFCIH VQCNASKFHQ GALLVAVLPE YVIGTVAGGT GTEDSHPPYK QTQPGADGFE L QHPYVLDA ...String: SPSAEACGYS DRVAQLTIGN STITTQEAAN IIVGYGEWPS YCSDSDATAV DKPTRPDVSV NRFYTLDTKL WEKSSKGWYW KFPDVLTET GVFGQNAQFH YLYRSGFCIH VQCNASKFHQ GALLVAVLPE YVIGTVAGGT GTEDSHPPYK QTQPGADGFE L QHPYVLDA GIPISQLTVC PHQWINLRTN NCATIIVPYI NALPFDSALN HCNFGLLVVP ISPLDYDQGA TPVIPITITL AP MCSEFAG LRQAVTQ UniProtKB: Genome polyprotein |
-Macromolecule #3: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Enterovirus A71 Enterovirus A71 |
| Molecular weight | Theoretical: 26.468225 KDa |
| Sequence | String: GFPTELKPGT NQFLTTDDGV SAPILPNFHP TPCIHIPGEV RNLLELCQVE TILEVNNVPT NATSLMERLR FPVSAQAGKG ELCAVFRAD PGRNGPWQST LLGQLCGYYT QWSGSLEVTF MFTGSFMATG KMLIAYTPPG GPLPKDRATA MLGTHVIWDF G LQSSVTLV ...String: GFPTELKPGT NQFLTTDDGV SAPILPNFHP TPCIHIPGEV RNLLELCQVE TILEVNNVPT NATSLMERLR FPVSAQAGKG ELCAVFRAD PGRNGPWQST LLGQLCGYYT QWSGSLEVTF MFTGSFMATG KMLIAYTPPG GPLPKDRATA MLGTHVIWDF G LQSSVTLV IPWISNTHYR AHARDGVFDY YTTGLVSIWY QTNYVVPIGA PNTAYIIALA AAQKNFTMKL CKDASDILQT GT IQ UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)