+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ATAT-2 bound K40Q MEC-12/MEC-7 microtubule | |||||||||
 Map data Map data | Major map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Microtubule / luminal enzyme / PTM / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of detection of mechanical stimulus involved in sensory perception of touch / positive regulation of mechanosensory behavior / COPI-mediated anterograde transport / COPI-independent Golgi-to-ER retrograde traffic / COPI-dependent Golgi-to-ER retrograde traffic / Kinesins / Intraflagellar transport / alpha-tubulin N-acetyltransferase / tubulin N-acetyltransferase activity / detection of mechanical stimulus involved in sensory perception of touch ...positive regulation of detection of mechanical stimulus involved in sensory perception of touch / positive regulation of mechanosensory behavior / COPI-mediated anterograde transport / COPI-independent Golgi-to-ER retrograde traffic / COPI-dependent Golgi-to-ER retrograde traffic / Kinesins / Intraflagellar transport / alpha-tubulin N-acetyltransferase / tubulin N-acetyltransferase activity / detection of mechanical stimulus involved in sensory perception of touch / detection of mechanical stimulus involved in sensory perception / thigmotaxis / L-lysine N-acetyltransferase activity, acting on acetyl phosphate as donor / mechanosensory behavior / response to mechanical stimulus / neuron development / microtubule-based process / cytoplasmic microtubule organization / regulation of microtubule cytoskeleton organization / structural constituent of cytoskeleton / microtubule cytoskeleton organization / mitotic cell cycle / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / microtubule / neuron projection / hydrolase activity / axon / neuronal cell body / GTPase activity / GTP binding / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Lam WH / Yu D / Zhai Y / Ti S | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: Tubulin acetyltransferases access and modify the microtubule luminal K40 residue through anchors in taxane-binding pockets. Authors: Jingyi Luo / Wai Hei Lam / Daqi Yu / Victor C Chao / Marc Nicholas Zopfi / Chen Jing Khoo / Chang Zhao / Shan Yan / Zheng Liu / Xiang David Li / Chaogu Zheng / Yuanliang Zhai / Shih-Chieh Ti /  Abstract: Acetylation at α-tubulin K40 is the sole post-translational modification preferred to occur inside the lumen of hollow cylindrical microtubules. However, how tubulin acetyltransferases access the ...Acetylation at α-tubulin K40 is the sole post-translational modification preferred to occur inside the lumen of hollow cylindrical microtubules. However, how tubulin acetyltransferases access the luminal K40 in micrometer-long microtubules remains unknown. Here, we use cryo-electron microscopy and single-molecule reconstitution assays to reveal the enzymatic mechanism for tubulin acetyltransferases to modify K40 in the lumen. One tubulin acetyltransferase spans across the luminal lattice, with the catalytic core docking onto two α-tubulins and the enzyme's C-terminal domain occupying the taxane-binding pockets of two β-tubulins. The luminal accessibility and enzyme processivity of tubulin acetyltransferases are inhibited by paclitaxel, a microtubule-stabilizing chemotherapeutic agent. Characterizations using recombinant tubulins mimicking preacetylated and postacetylated K40 show the crosstalk between microtubule acetylation states and the cofactor acetyl-CoA in enzyme turnover. Our findings provide crucial insights into the conserved multivalent interactions involving α- and β-tubulins to acetylate the confined microtubule lumen. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39102.map.gz emd_39102.map.gz | 121.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39102-v30.xml emd-39102-v30.xml emd-39102.xml emd-39102.xml | 26.9 KB 26.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39102_fsc.xml emd_39102_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_39102.png emd_39102.png | 44 KB | ||
| Filedesc metadata |  emd-39102.cif.gz emd-39102.cif.gz | 7.6 KB | ||
| Others |  emd_39102_additional_1.map.gz emd_39102_additional_1.map.gz emd_39102_half_map_1.map.gz emd_39102_half_map_1.map.gz emd_39102_half_map_2.map.gz emd_39102_half_map_2.map.gz | 230.1 MB 226.3 MB 226.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39102 http://ftp.pdbj.org/pub/emdb/structures/EMD-39102 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39102 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39102 | HTTPS FTP |
-Related structure data
| Related structure data |  8yalMC  8y9fC  8yajC  8yarC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39102.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39102.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Major map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
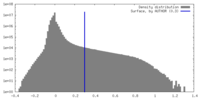
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Sharpened map
| File | emd_39102_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_39102_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_39102_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : K40Q MEC-12/MEC-7 microtubule complexed with alpha TAT2
| Entire | Name: K40Q MEC-12/MEC-7 microtubule complexed with alpha TAT2 |
|---|---|
| Components |
|
-Supramolecule #1: K40Q MEC-12/MEC-7 microtubule complexed with alpha TAT2
| Supramolecule | Name: K40Q MEC-12/MEC-7 microtubule complexed with alpha TAT2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Tubulin alpha-3 chain
| Macromolecule | Name: Tubulin alpha-3 chain / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 50.165367 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MREVISIHIG QAGVQIGNAC WELYCLEHGI QPDGQMPSDQ SLGGSDDSFS TFFSETGSGR HVPRAVMVDL EPTVIDEIRT GTYRSLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLTL DRIRRLADNC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKAKLE ...String: MREVISIHIG QAGVQIGNAC WELYCLEHGI QPDGQMPSDQ SLGGSDDSFS TFFSETGSGR HVPRAVMVDL EPTVIDEIRT GTYRSLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLTL DRIRRLADNC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKAKLE FSIYPAPQVS TAVVEPYNSI LTTHTTLEHS DCSFMVDNEA IYDICRRNLD IERPSYTNLN RLIGQIVSSI TA SLRFDGA LNVDLTEFQT NLVPYPRIHF PLATFSPVIS AEKAYHEQLS VAEITNMCFE PHNQMVKCDP RHGKYMAVCL LFR GDVVPK DVNAAIATIK TKRSIQFVDW CPTGFKVGIN YQPPTVVPGG DLAKVPRAVC MLSNTTAIAE AWARLDHKFD LMYA KRAFV HWYVGEGMEE GEFSEAREDL AALEKDYEEV GVDSMEDNGE EGDEY UniProtKB: Tubulin alpha-3 chain |
-Macromolecule #2: Alpha-tubulin N-acetyltransferase 2
| Macromolecule | Name: Alpha-tubulin N-acetyltransferase 2 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO / EC number: alpha-tubulin N-acetyltransferase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.434654 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MEIAFDLSTI FTDNIQRLTR TDLLKYGPKR YWAVAQSIDC LGEMSSKFHG WKRVITMYDK IVDHDEEQTT YIMWEKVNGS KSILKGLLR VGYKTLYLTD NEQNQYMEKA MCILDFFVVP TEQRSGNGFK MFDEMLKAEN VTVDQCAFDK PSAALQQFLE K YYDRKDLV ...String: MEIAFDLSTI FTDNIQRLTR TDLLKYGPKR YWAVAQSIDC LGEMSSKFHG WKRVITMYDK IVDHDEEQTT YIMWEKVNGS KSILKGLLR VGYKTLYLTD NEQNQYMEKA MCILDFFVVP TEQRSGNGFK MFDEMLKAEN VTVDQCAFDK PSAALQQFLE K YYDRKDLV WQSNKYALCS NFFIGRHPTV PFTPRQTKRA SRASSAVSSH ASSRNTSPIG RNRPRHDSVA DLMRQDMLAG VR AEVDPNS PTGLKNARDF GHRRIW UniProtKB: Alpha-tubulin N-acetyltransferase 2 |
-Macromolecule #3: Tubulin beta-1 chain
| Macromolecule | Name: Tubulin beta-1 chain / type: protein_or_peptide / ID: 3 / Details: MEC-7 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.306176 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MREIVHIQAG QCGNQIGSKF WEVISDEHGI DPSGQYVGDS DLQLERINVY YNEAGSNKYV PRAVLVDLEP GTMDSVRSGP FGQLFRPDN YVFGQSGAGN NWAKGHYTEG AELVDNVLDV VRKEAESTDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS ...String: MREIVHIQAG QCGNQIGSKF WEVISDEHGI DPSGQYVGDS DLQLERINVY YNEAGSNKYV PRAVLVDLEP GTMDSVRSGP FGQLFRPDN YVFGQSGAGN NWAKGHYTEG AELVDNVLDV VRKEAESTDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS VVPSPKVSDT VVEPYNATLS VHQLVENTDS TFCIDNEALY DICFRTLKLT TPTYGDLNHL VSATMSGVTT CL RFPGQLN ADLRKLAVNM VPFPRLHFFM PGFAPLTSRS NQQYRAITVP ELTQQCFDAK NMMAACDPRH GRYLTAAAIF RGR MSMKEV DEQMLNIQNK NSSYFVDWIP NNVKTAVCDI PPRGLKMSAT FIGNSTAIQE LFKRISEQFT AMFRRKAFLH WYTG EGMDE MEFTEAESNM NDLVSEYQQY QEAAADEDAA EAFDGE UniProtKB: Tubulin beta-1 chain |
-Macromolecule #4: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #5: ACETYL COENZYME *A
| Macromolecule | Name: ACETYL COENZYME *A / type: ligand / ID: 5 / Number of copies: 1 / Formula: ACO |
|---|---|
| Molecular weight | Theoretical: 809.571 Da |
| Chemical component information |  ChemComp-ACO: |
-Macromolecule #6: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER
| Macromolecule | Name: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER / type: ligand / ID: 6 / Number of copies: 2 / Formula: G2P |
|---|---|
| Molecular weight | Theoretical: 521.208 Da |
| Chemical component information |  ChemComp-G2P: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 2.2 µm / Calibrated defocus min: 1.1 µm / Calibrated magnification: 47170 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.1 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8yal: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)