[English] 日本語
 Yorodumi
Yorodumi- EMDB-38684: BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local refinement) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / Spike / SARS-CoV-2 / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell ...Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.69 Å | |||||||||
 Authors Authors | Yue C / Liu P | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Natl Sci Rev / Year: 2024 Journal: Natl Sci Rev / Year: 2024Title: Spike N354 glycosylation augments SARS-CoV-2 fitness for human adaptation through structural plasticity. Authors: Pan Liu / Can Yue / Bo Meng / Tianhe Xiao / Sijie Yang / Shuo Liu / Fanchong Jian / Qianhui Zhu / Yuanling Yu / Yanyan Ren / Peng Wang / Yixin Li / Jinyue Wang / Xin Mao / Fei Shao / Youchun ...Authors: Pan Liu / Can Yue / Bo Meng / Tianhe Xiao / Sijie Yang / Shuo Liu / Fanchong Jian / Qianhui Zhu / Yuanling Yu / Yanyan Ren / Peng Wang / Yixin Li / Jinyue Wang / Xin Mao / Fei Shao / Youchun Wang / Ravindra Kumar Gupta / Yunlong Cao / Xiangxi Wang /   Abstract: Selective pressures have given rise to a number of SARS-CoV-2 variants during the prolonged course of the COVID-19 pandemic. Recently evolved variants differ from ancestors in additional ...Selective pressures have given rise to a number of SARS-CoV-2 variants during the prolonged course of the COVID-19 pandemic. Recently evolved variants differ from ancestors in additional glycosylation within the spike protein receptor-binding domain (RBD). Details of how the acquisition of glycosylation impacts viral fitness and human adaptation are not clearly understood. Here, we dissected the role of N354-linked glycosylation, acquired by BA.2.86 sub-lineages, as a RBD conformational control element in attenuating viral infectivity. The reduced infectivity is recovered in the presence of heparin sulfate, which targets the 'N354 pocket' to ease restrictions of conformational transition resulting in a 'RBD-up' state, thereby conferring an adjustable infectivity. Furthermore, N354 glycosylation improved spike cleavage and cell-cell fusion, and in particular escaped one subset of ADCC antibodies. Together with reduced immunogenicity in hybrid immunity background, these indicate a single spike amino acid glycosylation event provides selective advantage in humans through multiple mechanisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38684.map.gz emd_38684.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38684-v30.xml emd-38684-v30.xml emd-38684.xml emd-38684.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38684.png emd_38684.png | 28.2 KB | ||
| Filedesc metadata |  emd-38684.cif.gz emd-38684.cif.gz | 6.3 KB | ||
| Others |  emd_38684_half_map_1.map.gz emd_38684_half_map_1.map.gz emd_38684_half_map_2.map.gz emd_38684_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38684 http://ftp.pdbj.org/pub/emdb/structures/EMD-38684 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38684 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38684 | HTTPS FTP |
-Related structure data
| Related structure data |  8xuuMC  8whsC  8whuC  8whvC  8whwC  8whzC  8x4hC  8x4zC  8x50C  8x55C  8x56C  8x5qC  8x5rC  8xurC  8xusC  8xutC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38684.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38684.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38684_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_38684_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local...
| Entire | Name: BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local refinement) |
|---|---|
| Components |
|
-Supramolecule #1: BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local...
| Supramolecule | Name: BA.2.86-T356K Spike Trimer in complex with heparan sulfate (Local refinement) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 134.069234 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ATMFVFLVLL PLVSSQCVMP LFNLITTTQS YTNSFTRGVY YPDKVFRSSV LHLTQDLFLP FFSNVTWFHA ISGTNGTKRF DNPVLPFND GVYFASTEKS NIIRGWIFGT TLDSKTQSLL IVNNATNVFI KVCEFQFCND PFLDVYHKNN KSWMESESGV Y SSANNCTF ...String: ATMFVFLVLL PLVSSQCVMP LFNLITTTQS YTNSFTRGVY YPDKVFRSSV LHLTQDLFLP FFSNVTWFHA ISGTNGTKRF DNPVLPFND GVYFASTEKS NIIRGWIFGT TLDSKTQSLL IVNNATNVFI KVCEFQFCND PFLDVYHKNN KSWMESESGV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PIIGRDFPQG FSALEPLVDL PIGINITRFQ TL LALNRSY LTPGDSSSGW TAGAADYYVG YLQPRTFLLK YNENGTITDA VDCALDPLSE TKCTLKSFTV EKGIYQTSNF RVQ PTESIV RFPNVTNLCP FHEVFNATRF ASVYAWNRKR ISNCVADYSV LYNFAPFFAF KCYGVSPTKL NDLCFTNVYA DSFV IKGNE VSQIAPGQTG NIADYNYKLP DDFTGCVIAW NSNKLDSKHS GNYDYWYRLF RKSKLKPFER DISTEIYQAG NKPCK GKGP NCYFPLQSYG FRPTYGVGHQ PYRVVVLSFE LLHAPATVCG PKKSTNLVKN KCVNFNFNGL TGTGVLTKSN KKFLPF QQF GRDIVDTTDA VRDPQTLEIL DITPCSFGGV SVITPGTNTS NQVAVLYQGV NCTEVSVAIH ADQLTPTWRV YSTGSNV FQ TRAGCLIGAE YVNNSYECDI PIGAGICASY QTQTKSRRAA ASVASQSIIA YTMSLGAENS VAYSNNSIAI PTNFTISV T TEILPVSMTK TSVDCTMYIC GDSTECSNLL LQYGSFCTQL KRALTGIAVE QDKNTQEVFA QVKQIYKTPP IKYFGGFNF SQILPDPSKP SKRSPIEDLL FNKVTLADAG FIKQYGDCLG DIAARDLICA QKFNGLTVLP PLLTDEMIAQ YTSALLAGTI TSGWTFGAG PALQIPFPMQ MAYRFNGIGV TQNVLYENQK LIANQFNSAI GKIQDSLFST PSALGKLQDV VNHNAQALNT L VKQLSSKF GAISSVLNDI LSRLDPPEAE VQIDRLITGR LQSLQTYVTQ QLIRAAEIRA SANLAATKMS ECVLGQSKRV DF CGKGYHL MSFPQSAPHG VVFLHVTYVP AQEKNFTTAP AICHDGKAHF PREGVFVSNG THWFVTQRNF YEPQIITTDN TFV SGNCDV VIGIVNNTVY DPLQLELDSF KEELDKYFKN HTSPDVDLGD ISGINASVVN IQKEIDRLNE VAKNLNESLI DLQE LGKYE Q UniProtKB: Spike glycoprotein |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: 2-O-sulfo-beta-L-altropyranuronic acid
| Macromolecule | Name: 2-O-sulfo-beta-L-altropyranuronic acid / type: ligand / ID: 4 / Number of copies: 1 / Formula: IDU |
|---|---|
| Molecular weight | Theoretical: 274.203 Da |
| Chemical component information | 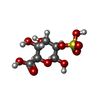 ChemComp-IDU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.69 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 315172 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































