[English] 日本語
 Yorodumi
Yorodumi- EMDB-38530: Cryo-EM structure of GPR30-Gq complex structure in the presence o... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of GPR30-Gq complex structure in the presence of fulvestrant | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / estrogen / GPR30 / Gq / MEMBRANE PROTEIN / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of leukocyte activation / nuclear fragmentation involved in apoptotic nuclear change / G protein-coupled estrogen receptor activity / positive regulation of cardiac vascular smooth muscle cell differentiation / negative regulation of cell cycle process / keratin filament / positive regulation of inositol trisphosphate biosynthetic process / apoptotic chromosome condensation / positive regulation of uterine smooth muscle contraction / negative regulation of lipid biosynthetic process ...negative regulation of leukocyte activation / nuclear fragmentation involved in apoptotic nuclear change / G protein-coupled estrogen receptor activity / positive regulation of cardiac vascular smooth muscle cell differentiation / negative regulation of cell cycle process / keratin filament / positive regulation of inositol trisphosphate biosynthetic process / apoptotic chromosome condensation / positive regulation of uterine smooth muscle contraction / negative regulation of lipid biosynthetic process / steroid hormone binding / cellular response to mineralocorticoid stimulus / positive regulation of G protein-coupled receptor signaling pathway / dendritic spine membrane / presynaptic active zone / positive regulation of extrinsic apoptotic signaling pathway / positive regulation of neurogenesis / cellular response to peptide hormone stimulus / nuclear estrogen receptor activity / dendritic spine head / steroid hormone receptor signaling pathway / positive regulation of neurotransmitter secretion / negative regulation of fat cell differentiation / positive regulation of release of cytochrome c from mitochondria / negative regulation of vascular associated smooth muscle cell proliferation / positive regulation of endothelial cell apoptotic process / nuclear receptor-mediated steroid hormone signaling pathway / regulation of cytosolic calcium ion concentration / positive regulation of epidermal growth factor receptor signaling pathway / neuronal action potential / steroid binding / negative regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / axon terminus / cytoplasmic vesicle membrane / Peptide ligand-binding receptors / positive regulation of release of sequestered calcium ion into cytosol / hippocampal mossy fiber to CA3 synapse / dendritic shaft / positive regulation of protein localization to plasma membrane / cellular response to estradiol stimulus / trans-Golgi network / cellular response to glucose stimulus / positive regulation of insulin secretion / recycling endosome / negative regulation of ERK1 and ERK2 cascade / G protein-coupled receptor activity / negative regulation of inflammatory response / mitochondrial membrane / vasodilation / Olfactory Signaling Pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of protein phosphorylation / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / cellular response to tumor necrosis factor / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / nuclear envelope / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / nervous system development / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / positive regulation of cytosolic calcium ion concentration / presynaptic membrane / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Liu H / Xu P / Xu HE | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2024 Journal: Cell Res / Year: 2024Title: Structural and functional evidence that GPR30 is not a direct estrogen receptor. Authors: Heng Liu / Shimeng Guo / Antao Dai / Peiyu Xu / Xin Li / Sijie Huang / Xinheng He / Kai Wu / Xinyue Zhang / Dehua Yang / Xin Xie / H Eric Xu /  | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38530.map.gz emd_38530.map.gz | 28.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38530-v30.xml emd-38530-v30.xml emd-38530.xml emd-38530.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38530.png emd_38530.png | 40.8 KB | ||
| Masks |  emd_38530_msk_1.map emd_38530_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-38530.cif.gz emd-38530.cif.gz | 7.2 KB | ||
| Others |  emd_38530_half_map_1.map.gz emd_38530_half_map_1.map.gz emd_38530_half_map_2.map.gz emd_38530_half_map_2.map.gz | 23.3 MB 23.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38530 http://ftp.pdbj.org/pub/emdb/structures/EMD-38530 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38530 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38530 | HTTPS FTP |
-Related structure data
| Related structure data |  8xoiMC  8xofC  8xogC  8xohC  8xojC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38530.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38530.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_38530_msk_1.map emd_38530_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
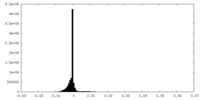
| Density Histograms |
-Half map: #2
| File | emd_38530_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
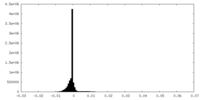
| Density Histograms |
-Half map: #1
| File | emd_38530_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
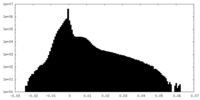
| Density Histograms |
- Sample components
Sample components
-Entire : GPR30-Gq complex
| Entire | Name: GPR30-Gq complex |
|---|---|
| Components |
|
-Supramolecule #1: GPR30-Gq complex
| Supramolecule | Name: GPR30-Gq complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: GPR30-Gq
| Supramolecule | Name: GPR30-Gq / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2, #4-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: ScFv16
| Supramolecule | Name: ScFv16 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: Guanine nucleotide-binding protein G(q) subunit alpha-q
| Macromolecule | Name: Guanine nucleotide-binding protein G(q) subunit alpha-q type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.632219 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RRTLRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RRTLRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTSGIFETK FQVDKVNFHM FDVGAQRDER RKWIQCFNDV TAIIFVVDSS DTNRLQEALN DF DSIWNNR WLRTISVILF LNKQDLLAEK VLAGKSKIED YFPEFARYTT PEDATPEPGE DPRVTRAKYF IRKEFVDIST ASG DGRHIC YPHFTCAVDT ENARRIFNDC KDIILQMNLR EYNLV |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.744371 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 26.293299 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: G-protein coupled estrogen receptor 1
| Macromolecule | Name: G-protein coupled estrogen receptor 1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.291457 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDVTSQARGV GLEMYPGTAQ PAAPNTTSPE LNLSHPLLGT ALANGTGELS EHQQYVIGLF LSCLYTIFLF PIGFVGNILI LVVNISFRE KMTIPDLYFI NLAVADLILV ADSLIEVFNL HERYYDIAVL CTFMSLFLQV NMYSSVFFLT WMSFDRYIAL A RAMRCSLF ...String: MDVTSQARGV GLEMYPGTAQ PAAPNTTSPE LNLSHPLLGT ALANGTGELS EHQQYVIGLF LSCLYTIFLF PIGFVGNILI LVVNISFRE KMTIPDLYFI NLAVADLILV ADSLIEVFNL HERYYDIAVL CTFMSLFLQV NMYSSVFFLT WMSFDRYIAL A RAMRCSLF RTKHHARLSC GLIWMASVSA TLVPFTAVHL QHTDEACFCF ADVREVQWLE VTLGFIVPFA IIGLCYSLIV RV LVRAHRH RGLRPRRQKA LRMILAVVLV FFVCWLPENV FISVHLLQRT QPGAAPCKQS FRHAHPLTGH IVNLAAFSNS CLN PLIYSF LGETFRDKLR LYIEQKTNLP ALNRFCHAAL KAVIPDSTEQ SDVRFSSAV UniProtKB: G-protein coupled estrogen receptor 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)