[English] 日本語
 Yorodumi
Yorodumi- EMDB-38291: Cryo-EM structure of human XKR8-basigin complex in lipid nanodisc -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human XKR8-basigin complex in lipid nanodisc | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | scramblase / apoptosis / membrane protein / LIPID TRANSPORT | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationDefective SLC16A1 causes symptomatic deficiency in lactate transport (SDLT) / Proton-coupled monocarboxylate transport / phosphatidylserine exposure on apoptotic cell surface / tolerance induction to self antigen / positive regulation of matrix metallopeptidase secretion / acrosomal membrane / neutrophil clearance / dendrite self-avoidance / phospholipid scramblase activity / endothelial tube morphogenesis ...Defective SLC16A1 causes symptomatic deficiency in lactate transport (SDLT) / Proton-coupled monocarboxylate transport / phosphatidylserine exposure on apoptotic cell surface / tolerance induction to self antigen / positive regulation of matrix metallopeptidase secretion / acrosomal membrane / neutrophil clearance / dendrite self-avoidance / phospholipid scramblase activity / endothelial tube morphogenesis / cell-cell adhesion mediator activity / response to mercury ion / engulfment of apoptotic cell / apoptotic process involved in development / neural retina development / photoreceptor cell maintenance / Basigin interactions / Aspirin ADME / odontogenesis of dentin-containing tooth / D-mannose binding / homophilic cell-cell adhesion / decidualization / positive regulation of vascular endothelial growth factor production / photoreceptor outer segment / Integrin cell surface interactions / response to cAMP / positive regulation of myoblast differentiation / Degradation of the extracellular matrix / neutrophil chemotaxis / photoreceptor inner segment / positive regulation of endothelial cell migration / embryo implantation / axon guidance / protein localization to plasma membrane / establishment of localization in cell / sarcolemma / positive regulation of interleukin-6 production / response to peptide hormone / melanosome / signaling receptor activity / virus receptor activity / angiogenesis / basolateral plasma membrane / positive regulation of viral entry into host cell / cell surface receptor signaling pathway / endosome / cadherin binding / Golgi membrane / axon / focal adhesion / endoplasmic reticulum membrane / perinuclear region of cytoplasm / mitochondrion / extracellular exosome / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.66 Å | ||||||||||||
 Authors Authors | Sakuragi TS / Kanai RK / Kikkawa MK / Toyoshima CT / Nagata SN | ||||||||||||
| Funding support |  Japan, 3 items Japan, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: The role of the C-terminal tail region as a plug to regulate XKR8 lipid scramblase. Authors: Takaharu Sakuragi / Ryuta Kanai / Mayumi Otani / Masahide Kikkawa / Chikashi Toyoshima / Shigekazu Nagata /  Abstract: XK-related 8 (XKR8), in complex with the transmembrane glycoprotein basigin, functions as a phospholipid scramblase activated by the caspase-mediated cleavage or phosphorylation of its C-terminal ...XK-related 8 (XKR8), in complex with the transmembrane glycoprotein basigin, functions as a phospholipid scramblase activated by the caspase-mediated cleavage or phosphorylation of its C-terminal tail. It carries a putative phospholipid translocation path of multiple hydrophobic and charged residues in the transmembrane region. It also has a crucial tryptophan at the exoplasmic end of the path that regulates its scrambling activity. We herein investigated the tertiary structure of the human XKR8-basigin complex embedded in lipid nanodiscs at an overall resolution of 3.66 Å. We found that the C-terminal tail engaged in intricate polar and van der Waals interactions with a groove at the cytoplasmic surface of XKR8. These interactions maintained the inactive state of XKR8. Point mutations to disrupt these interactions strongly enhanced the scrambling activity of XKR8, suggesting that the activation of XKR8 is mediated by releasing the C-terminal tail from the cytoplasmic groove. We speculate that the cytoplasmic tail region of XKR8 functions as a plug to prevent the scrambling of phospholipids. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38291.map.gz emd_38291.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38291-v30.xml emd-38291-v30.xml emd-38291.xml emd-38291.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38291.png emd_38291.png | 96.4 KB | ||
| Filedesc metadata |  emd-38291.cif.gz emd-38291.cif.gz | 6.3 KB | ||
| Others |  emd_38291_half_map_1.map.gz emd_38291_half_map_1.map.gz emd_38291_half_map_2.map.gz emd_38291_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38291 http://ftp.pdbj.org/pub/emdb/structures/EMD-38291 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38291 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38291 | HTTPS FTP |
-Related structure data
| Related structure data |  8xejMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_38291.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38291.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.23203 Å | ||||||||||||||||||||||||||||||||||||
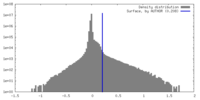
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_38291_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
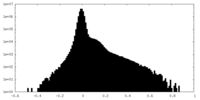
| Projections & Slices |
| ||||||||||||
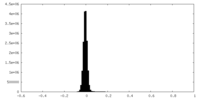
| Density Histograms |
-Half map: #2
| File | emd_38291_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
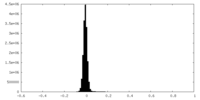
| Density Histograms |
- Sample components
Sample components
-Entire : human XKR8-basigin complex bound to Fab fragment in lipid nanodisc
| Entire | Name: human XKR8-basigin complex bound to Fab fragment in lipid nanodisc |
|---|---|
| Components |
|
-Supramolecule #1: human XKR8-basigin complex bound to Fab fragment in lipid nanodisc
| Supramolecule | Name: human XKR8-basigin complex bound to Fab fragment in lipid nanodisc type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Molecular weight | Theoretical: 110 KDa |
-Supramolecule #2: human XKR8-basigin complex
| Supramolecule | Name: human XKR8-basigin complex / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Fab fragment
| Supramolecule | Name: Fab fragment / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3-#4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Isoform 2 of Basigin
| Macromolecule | Name: Isoform 2 of Basigin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 19.592814 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AGPPRVKAVK SSEHINEGET AMLVCKSESV PPVTDWAWYK ITDSEDKALM QGSESRFFVS SSQGRSELHI ENLNMEADPG QYRCQGTSS KGSDQAIITL RVRSHLAALW PFLGIVAEVL VLVTIIFIYE KRRKPEDVLD DDDAGSAPLK SSGQHQNDKG K NVRQRNSS DYKDDDDK UniProtKB: Basigin |
-Macromolecule #2: XK-related protein 8
| Macromolecule | Name: XK-related protein 8 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.975609 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPWSSRGALL RDLVLGVLGT AAFLLDLGTD LWAAVQYALG GRYLWAALVL ALLGLASVAL QLFSWLWLRA DPAGLHGSQP PRRCLALLH LLQLGYLYRC VQELRQGLLV WQQEEPSEFD LAYADFLALD ISMLRLFETF LETAPQLTLV LAIMLQSGRA E YYQWVGIC ...String: MPWSSRGALL RDLVLGVLGT AAFLLDLGTD LWAAVQYALG GRYLWAALVL ALLGLASVAL QLFSWLWLRA DPAGLHGSQP PRRCLALLH LLQLGYLYRC VQELRQGLLV WQQEEPSEFD LAYADFLALD ISMLRLFETF LETAPQLTLV LAIMLQSGRA E YYQWVGIC TSFLGISWAL LDYHRALRTC LPSKPLLGLG SSVIYFLWNL LLLWPRVLAV ALFSALFPSY VALHFLGLWL VL LLWVWLQ GTDFMPDPSS EWLYRVTVAT ILYFSWFNVA EGRTRGRAII HFAFLLSDSI LLVATWVTHS SWLPSGIPLQ LWL PVGCGC FFLGLALRLV YYHWLHPSCC WKPDPDQVDG ARSLLSPEGY QLPQNRRMTH LAQKFFPKAK DEAASPVKGV DEFE NLYFQ UniProtKB: XK-related protein 8 |
-Macromolecule #3: Fab heavy chain
| Macromolecule | Name: Fab heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.869639 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: (PCA)SVEESGGRL VTPGTPLTLT CTVSGFSLSD YAMNWVRQAP GKGLEWIGII YASGSRYYAS WAKGRFTISK TSTTVD LKI TSPTTEDTAT YFCARYYAGS DIWGPGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV TVSWNSG AL TSGVHTFPAV ...String: (PCA)SVEESGGRL VTPGTPLTLT CTVSGFSLSD YAMNWVRQAP GKGLEWIGII YASGSRYYAS WAKGRFTISK TSTTVD LKI TSPTTEDTAT YFCARYYAGS DIWGPGTLVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV TVSWNSG AL TSGVHTFPAV LQSSGLYSLS SVVTVPSSSL GTQTYICNVN HKPSNTKVDK KVEPKSCDK |
-Macromolecule #4: Fab light chain
| Macromolecule | Name: Fab light chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.261865 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: ADVVMTQTPS SVSAAVGGTV TINCQASQSI SAYLAWYQQK PGQPPKLLIY DASDLASGVS SRFKGSGSGT QFTLTISALE CADAATYYC QSYYAIITYG AAFGGGTEVV VKRTVAAPSV FIFPPSDEQL KSGTASVVCL LNNFYPREAK VQWKVDNALQ S GNSQESVT ...String: ADVVMTQTPS SVSAAVGGTV TINCQASQSI SAYLAWYQQK PGQPPKLLIY DASDLASGVS SRFKGSGSGT QFTLTISALE CADAATYYC QSYYAIITYG AAFGGGTEVV VKRTVAAPSV FIFPPSDEQL KSGTASVVCL LNNFYPREAK VQWKVDNALQ S GNSQESVT EQDSKDCTYS LSSTLTLSKA DYEKHKVYAC EVTHQGLSSP VTKSFNRGEC |
-Macromolecule #5: 1,2-DILINOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DILINOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 5 / Number of copies: 1 / Formula: DLP |
|---|---|
| Molecular weight | Theoretical: 782.082 Da |
| Chemical component information |  ChemComp-DLP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.66 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 162528 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)