[English] 日本語
 Yorodumi
Yorodumi- EMDB-37627: Cryo-EM structure of the distal rod-hook within the flagellar mot... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the distal rod-hook within the flagellar motor-hook complex in the CCW state. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Flagellum / Flagellar motor / MOTOR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial-type flagellum basal body, distal rod / bacterial-type flagellum hook / bacterial-type flagellum basal body / bacterial-type flagellum-dependent swarming motility / bacterial-type flagellum / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) | ||||||||||||
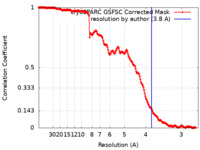
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Tan JX / Zhang L / Zhou Y / Zhu YQ | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell Res / Year: 2024 Journal: Cell Res / Year: 2024Title: Structural basis of the bacterial flagellar motor rotational switching. Authors: Jiaxing Tan / Ling Zhang / Xingtong Zhou / Siyu Han / Yan Zhou / Yongqun Zhu /  Abstract: The bacterial flagellar motor is a huge bidirectional rotary nanomachine that drives rotation of the flagellum for bacterial motility. The cytoplasmic C ring of the flagellar motor functions as the ...The bacterial flagellar motor is a huge bidirectional rotary nanomachine that drives rotation of the flagellum for bacterial motility. The cytoplasmic C ring of the flagellar motor functions as the switch complex for the rotational direction switching from counterclockwise to clockwise. However, the structural basis of the rotational switching and how the C ring is assembled have long remained elusive. Here, we present two high-resolution cryo-electron microscopy structures of the C ring-containing flagellar basal body-hook complex from Salmonella Typhimurium, which are in the default counterclockwise state and in a constitutively active CheY mutant-induced clockwise state, respectively. In both complexes, the C ring consists of four subrings, but is in two different conformations. The CheY proteins are bound into an open groove between two adjacent protomers on the surface of the middle subring of the C ring and interact with the FliG and FliM subunits. The binding of the CheY protein induces a significant upward shift of the C ring towards the MS ring and inward movements of its protomers towards the motor center, which eventually remodels the structures of the FliG subunits and reverses the orientations and surface electrostatic potential of the α helices to trigger the counterclockwise-to-clockwise rotational switching. The conformational changes of the FliG subunits reveal that the stator units on the motor require a relocation process in the inner membrane during the rotational switching. This study provides unprecedented molecular insights into the rotational switching mechanism and a detailed overall structural view of the bacterial flagellar motors. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37627.map.gz emd_37627.map.gz | 483.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37627-v30.xml emd-37627-v30.xml emd-37627.xml emd-37627.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37627_fsc.xml emd_37627_fsc.xml | 17.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_37627.png emd_37627.png | 84.9 KB | ||
| Filedesc metadata |  emd-37627.cif.gz emd-37627.cif.gz | 6.4 KB | ||
| Others |  emd_37627_half_map_1.map.gz emd_37627_half_map_1.map.gz emd_37627_half_map_2.map.gz emd_37627_half_map_2.map.gz | 475.6 MB 475.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37627 http://ftp.pdbj.org/pub/emdb/structures/EMD-37627 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37627 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37627 | HTTPS FTP |
-Related structure data
| Related structure data |  8wlpMC  8whtC  8wiwC  8wjrC  8wk3C  8wk4C  8wkiC  8wkkC  8wkqC  8wl2C  8wleC  8wlhC  8wliC  8wlnC  8wlqC  8wltC  8wo5C  8woeC  8xp0C  8xp1C  8yjtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37627.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37627.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.332 Å | ||||||||||||||||||||||||||||||||||||
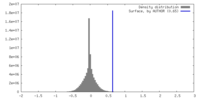
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_37627_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
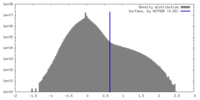
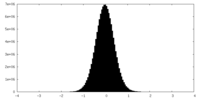
| Density Histograms |
-Half map: #2
| File | emd_37627_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
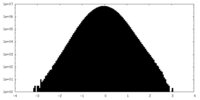
| Density Histograms |
- Sample components
Sample components
-Entire : C ring-containing flagellar motor-hook complex in the CCW state
| Entire | Name: C ring-containing flagellar motor-hook complex in the CCW state |
|---|---|
| Components |
|
-Supramolecule #1: C ring-containing flagellar motor-hook complex in the CCW state
| Supramolecule | Name: C ring-containing flagellar motor-hook complex in the CCW state type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) |
-Macromolecule #1: Flagellar basal-body rod protein FlgG
| Macromolecule | Name: Flagellar basal-body rod protein FlgG / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) |
| Molecular weight | Theoretical: 27.784807 KDa |
| Sequence | String: MISSLWIAKT GLDAQQTNMD VIANNLANVS TNGFKRQRAV FEDLLYQTIR QPGAQSSEQT TLPSGLQIGT GVRPVATERL HSQGNLSQT NNSKDVAIKG QGFFQVMLPD GTSAYTRDGS FQVDQNGQLV TAGGFQVQPA ITIPANALSI TIGRDGVVSV T QQGQAAPV ...String: MISSLWIAKT GLDAQQTNMD VIANNLANVS TNGFKRQRAV FEDLLYQTIR QPGAQSSEQT TLPSGLQIGT GVRPVATERL HSQGNLSQT NNSKDVAIKG QGFFQVMLPD GTSAYTRDGS FQVDQNGQLV TAGGFQVQPA ITIPANALSI TIGRDGVVSV T QQGQAAPV QVGQLNLTTF MNDTGLESIG ENLYIETQSS GAPNESTPGL NGAGLLYQGY VETSNVNVAE ELVNMIQVQR AY EINSKAV STTDQMLQKL TQL UniProtKB: Flagellar basal-body rod protein FlgG |
-Macromolecule #2: Flagellar hook protein FlgE
| Macromolecule | Name: Flagellar hook protein FlgE / type: protein_or_peptide / ID: 2 / Number of copies: 29 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) |
| Molecular weight | Theoretical: 42.233152 KDa |
| Sequence | String: MSFSQAVSGL NAAATNLDVI GNNIANSATY GFKSGTASFA DMFAGSKVGL GVKVAGITQD FTDGTTTNTG RGLDVAISQN GFFRLVDSN GSVFYSRNGQ FKLDENRNLV NMQGMQLTGY PATGTPPTIQ QGANPAPITI PNTLMAAKST TTASMQINLN S TDPVPSKT ...String: MSFSQAVSGL NAAATNLDVI GNNIANSATY GFKSGTASFA DMFAGSKVGL GVKVAGITQD FTDGTTTNTG RGLDVAISQN GFFRLVDSN GSVFYSRNGQ FKLDENRNLV NMQGMQLTGY PATGTPPTIQ QGANPAPITI PNTLMAAKST TTASMQINLN S TDPVPSKT PFSVSDADSY NKKGTVTVYD SQGNAHDMNV YFVKTKDNEW AVYTHDSSDP AATAPTTAST TLKFNENGIL ES GGTVNIT TGTINGATAA TFSLSFLNSM QQNTGANNIV ATNQNGYKPG DLVSYQINND GTVVGNYSNE QEQVLGQIVL ANF ANNEGL ASQGDNVWAA TQASGVALLG TAGSGNFGKL TNGALEASNV DLSKELVNMI VAQRNYQSNA QTIKTQDQIL NTLV NLR UniProtKB: Flagellar hook protein FlgE |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Blot time: 2 Blot force: -15 Wait time: 60 Blot total: 1. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: Other / Chain - Initial model type: in silico model / Details: Model-Angelo |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-8wlp: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)