[日本語] English
 万見
万見- EMDB-3713: 2.9 A cryo-EM structure of VemP-stalled ribosome-nascent chain complex -
+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-3713 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | 2.9 A cryo-EM structure of VemP-stalled ribosome-nascent chain complex | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | VemP-SRC / peptidyltransferase center / ribosomal exit tunnel / helix-loop-helix. ribosome / 70S / ribosome stalling / arrest peptide / ribosome | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of cytoplasmic translational initiation / stringent response / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis ...negative regulation of cytoplasmic translational initiation / stringent response / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / positive regulation of RNA splicing / ribosome assembly / transcription elongation factor complex / regulation of DNA-templated transcription elongation / cytosolic ribosome assembly / response to reactive oxygen species / DNA endonuclease activity / transcription antitermination / translational initiation / regulation of cell growth / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / ribosome biogenesis / large ribosomal subunit / regulation of translation / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / small ribosomal subunit rRNA binding / ribosomal large subunit assembly / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Vibrio alginolyticus (バクテリア) / Vibrio alginolyticus (バクテリア) /    | |||||||||
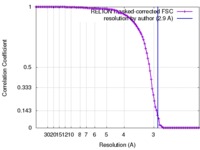
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.9 Å | |||||||||
 データ登録者 データ登録者 | Su T | |||||||||
 引用 引用 |  ジャーナル: Elife / 年: 2017 ジャーナル: Elife / 年: 2017タイトル: The force-sensing peptide VemP employs extreme compaction and secondary structure formation to induce ribosomal stalling. 著者: Ting Su / Jingdong Cheng / Daniel Sohmen / Rickard Hedman / Otto Berninghausen / Gunnar von Heijne / Daniel N Wilson / Roland Beckmann /   要旨: Interaction between the nascent polypeptide chain and the ribosomal exit tunnel can modulate the rate of translation and induce translational arrest to regulate expression of downstream genes. The ...Interaction between the nascent polypeptide chain and the ribosomal exit tunnel can modulate the rate of translation and induce translational arrest to regulate expression of downstream genes. The ribosomal tunnel also provides a protected environment for initial protein folding events. Here, we present a 2.9 Å cryo-electron microscopy structure of a ribosome stalled during translation of the extremely compacted VemP nascent chain. The nascent chain forms two α-helices connected by an α-turn and a loop, enabling a total of 37 amino acids to be observed within the first 50-55 Å of the exit tunnel. The structure reveals how α-helix formation directly within the peptidyltransferase center of the ribosome interferes with aminoacyl-tRNA accommodation, suggesting that during canonical translation, a major role of the exit tunnel is to prevent excessive secondary structure formation that can interfere with the peptidyltransferase activity of the ribosome. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_3713.map.gz emd_3713.map.gz | 175.2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-3713-v30.xml emd-3713-v30.xml emd-3713.xml emd-3713.xml | 77.3 KB 77.3 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_3713_fsc.xml emd_3713_fsc.xml | 10.4 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_3713.png emd_3713.png | 264.3 KB | ||
| Filedesc metadata |  emd-3713.cif.gz emd-3713.cif.gz | 13.4 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3713 http://ftp.pdbj.org/pub/emdb/structures/EMD-3713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3713 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_3713_validation.pdf.gz emd_3713_validation.pdf.gz | 639.1 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_3713_full_validation.pdf.gz emd_3713_full_validation.pdf.gz | 638.6 KB | 表示 | |
| XML形式データ |  emd_3713_validation.xml.gz emd_3713_validation.xml.gz | 12.2 KB | 表示 | |
| CIF形式データ |  emd_3713_validation.cif.gz emd_3713_validation.cif.gz | 16.1 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3713 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3713 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3713 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3713 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_3713.map.gz / 形式: CCP4 / 大きさ: 190.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_3713.map.gz / 形式: CCP4 / 大きさ: 190.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : VemP-stalled ribosome-nascent chain complex
+超分子 #1: VemP-stalled ribosome-nascent chain complex
+超分子 #2: VemP-stalled ribosome-nascent chain complex
+超分子 #3: VemP-stalled ribosome-nascent chain complex
+超分子 #4: VemP-stalled ribosome-nascent chain complex
+分子 #1: VemP nascent chain
+分子 #4: 50S ribosomal protein L2
+分子 #5: 50S ribosomal protein L3
+分子 #6: 50S ribosomal protein L4
+分子 #7: 50S ribosomal protein L5
+分子 #8: 50S ribosomal protein L6
+分子 #9: 50S ribosomal protein L9
+分子 #10: 50S ribosomal protein L11
+分子 #11: 50S ribosomal protein L13
+分子 #12: 50S ribosomal protein L14
+分子 #13: 50S ribosomal protein L15
+分子 #14: 50S ribosomal protein L16
+分子 #15: 50S ribosomal protein L17
+分子 #16: 50S ribosomal protein L18
+分子 #17: 50S ribosomal protein L19
+分子 #18: 50S ribosomal protein L20
+分子 #19: 50S ribosomal protein L21
+分子 #20: 50S ribosomal protein L22
+分子 #21: 50S ribosomal protein L23
+分子 #22: 50S ribosomal protein L24
+分子 #23: 50S ribosomal protein L25
+分子 #24: 50S ribosomal protein L27
+分子 #25: 50S ribosomal protein L28
+分子 #26: 50S ribosomal protein L29
+分子 #27: 50S ribosomal protein L30
+分子 #28: 50S ribosomal protein L32
+分子 #29: 50S ribosomal protein L33
+分子 #30: 50S ribosomal protein L34
+分子 #31: 50S ribosomal protein L35
+分子 #32: 50S ribosomal protein L36
+分子 #34: 50S ribosomal protein L31
+分子 #36: 30S ribosomal protein S2
+分子 #37: 30S ribosomal protein S3
+分子 #38: 30S ribosomal protein S4
+分子 #39: 30S ribosomal protein S5
+分子 #40: 30S ribosomal protein S6
+分子 #41: 30S ribosomal protein S7
+分子 #42: 30S ribosomal protein S8
+分子 #43: 30S ribosomal protein S9
+分子 #44: 30S ribosomal protein S10
+分子 #45: 30S ribosomal protein S11
+分子 #46: 30S ribosomal protein S12
+分子 #47: 30S ribosomal protein S13
+分子 #48: 30S ribosomal protein S14
+分子 #49: 30S ribosomal protein S15
+分子 #50: 30S ribosomal protein S16
+分子 #51: 30S ribosomal protein S17
+分子 #52: 30S ribosomal protein S18
+分子 #53: 30S ribosomal protein S19
+分子 #54: 30S ribosomal protein S20
+分子 #55: 30S ribosomal protein S21
+分子 #2: Gln-tRNA
+分子 #3: 5S rRNA
+分子 #33: 23S rRNA
+分子 #35: 16S rRNA
+分子 #56: mRNA
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.2 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: FEI FALCON II (4k x 4k) 平均電子線量: 2.4 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | プロトコル: RIGID BODY FIT |
|---|---|
| 得られたモデル |  PDB-5nwy: |
 ムービー
ムービー コントローラー
コントローラー


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)