[English] 日本語
 Yorodumi
Yorodumi- EMDB-36901: Cryo-EM structure of Acipimox bound human hydroxy-carboxylic acid... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Acipimox bound human hydroxy-carboxylic acid receptor 2 (Local refinement) | |||||||||
 Map data Map data | sharpening map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / G-Protein / MEMBRANE PROTEIN / acipimox / signaling | |||||||||
| Function / homology |  Function and homology information Function and homology informationneutrophil apoptotic process / nicotinic acid receptor activity / positive regulation of neutrophil apoptotic process / Hydroxycarboxylic acid-binding receptors / Class A/1 (Rhodopsin-like receptors) / positive regulation of adiponectin secretion / negative regulation of lipid catabolic process / cell junction / G alpha (i) signalling events / G protein-coupled receptor signaling pathway / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.13 Å | |||||||||
 Authors Authors | Park JH / Ishimoto N / Park SY | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis for ligand recognition and signaling of hydroxy-carboxylic acid receptor 2. Authors: Jae-Hyun Park / Kouki Kawakami / Naito Ishimoto / Tatsuya Ikuta / Mio Ohki / Toru Ekimoto / Mitsunori Ikeguchi / Dong-Sun Lee / Young-Ho Lee / Jeremy R H Tame / Asuka Inoue / Sam-Yong Park /   Abstract: Hydroxycarboxylic acid receptors (HCAR1, HCAR2, and HCAR3) transduce G signaling upon biding to molecules such as lactic acid, butyric acid and 3-hydroxyoctanoic acid, which are associated with ...Hydroxycarboxylic acid receptors (HCAR1, HCAR2, and HCAR3) transduce G signaling upon biding to molecules such as lactic acid, butyric acid and 3-hydroxyoctanoic acid, which are associated with lipolytic and atherogenic activity, and neuroinflammation. Although many reports have elucidated the function of HCAR2 and its potential as a therapeutic target for treating not only dyslipidemia but also neuroimmune disorders such as multiple sclerosis and Parkinson's disease, the structural basis of ligand recognition and ligand-induced G-coupling remains unclear. Here we report three cryo-EM structures of the human HCAR2-G signaling complex, each bound with different ligands: niacin, acipimox or GSK256073. All three agonists are held in a deep pocket lined by residues that are not conserved in HCAR1 and HCAR3. A distinct hairpin loop at the HCAR2 N-terminus and extra-cellular loop 2 (ECL2) completely enclose the ligand. These structures also reveal the agonist-induced conformational changes propagated to the G-protein-coupling interface during activation. Collectively, the structures presented here are expected to help in the design of ligands specific for HCAR2, leading to new drugs for the treatment of various diseases such as dyslipidemia and inflammation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36901.map.gz emd_36901.map.gz | 28.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36901-v30.xml emd-36901-v30.xml emd-36901.xml emd-36901.xml | 18.6 KB 18.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36901_fsc.xml emd_36901_fsc.xml | 6.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_36901.png emd_36901.png | 48.9 KB | ||
| Masks |  emd_36901_msk_1.map emd_36901_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-36901.cif.gz emd-36901.cif.gz | 5.9 KB | ||
| Others |  emd_36901_additional_1.map.gz emd_36901_additional_1.map.gz emd_36901_half_map_1.map.gz emd_36901_half_map_1.map.gz emd_36901_half_map_2.map.gz emd_36901_half_map_2.map.gz | 15.4 MB 28.4 MB 28.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36901 http://ftp.pdbj.org/pub/emdb/structures/EMD-36901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36901 | HTTPS FTP |
-Related structure data
| Related structure data |  8k5cMC  8i7vC  8i7wC  8k5bC  8k5dC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36901.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36901.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpening map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.245 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_36901_msk_1.map emd_36901_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: map
| File | emd_36901_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_36901_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1
| File | emd_36901_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Acipimox bound human hydroxy-carboxylic acid receptor 2
| Entire | Name: Acipimox bound human hydroxy-carboxylic acid receptor 2 |
|---|---|
| Components |
|
-Supramolecule #1: Acipimox bound human hydroxy-carboxylic acid receptor 2
| Supramolecule | Name: Acipimox bound human hydroxy-carboxylic acid receptor 2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 150 KDa |
-Macromolecule #1: Human hydroxycarboxylic acid receptor 2
| Macromolecule | Name: Human hydroxycarboxylic acid receptor 2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.320297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPGAPADLED NWETLNDNLK VIEKADNAAQ VKDALTKMRA AALDAQKATP PKLEDKSPDS PEMKDFRHGF DILVGQIDDA LKLANEGKV KEAQAAAEQL KTTRNAYIQK YLEFMNRHHL QDHFLEIDKK NCCVFRDDFI VKVLPPVLGL EFIFGLLGNG L ALWIFCFH ...String: GPGAPADLED NWETLNDNLK VIEKADNAAQ VKDALTKMRA AALDAQKATP PKLEDKSPDS PEMKDFRHGF DILVGQIDDA LKLANEGKV KEAQAAAEQL KTTRNAYIQK YLEFMNRHHL QDHFLEIDKK NCCVFRDDFI VKVLPPVLGL EFIFGLLGNG L ALWIFCFH LKSWKSSRIF LFNLAVADFL LIICLPFLMD NYVRRWDWKF GDIPCRLMLF MLAMNRQGSI IFLTVVAVDR YF RVVHPHH ALNKISNRTA AIISCLLWGI TIGLTVHLLK KKMPIQNGGA NLCSSFSICH TFQWHEAMFL LEFFLPLGII LFC SARIIW SLRQRQMDRH AKIKRAITFI MVVAIVFVIC FLPSVVVRIR IFWLLHTSGT QNCEVYRSVD LAFFITLSFT YMNS MLDPV VYYFSSPSFP NFFSTLINRC LQRKMTGEPD NNRSTSVELT GDPNKTRGAP EALMANSGEP WSPSYLGPTS P UniProtKB: Hydroxycarboxylic acid receptor 2 |
-Macromolecule #2: 5-methyl-4-oxidanyl-pyrazin-4-ium-2-carboxylic acid
| Macromolecule | Name: 5-methyl-4-oxidanyl-pyrazin-4-ium-2-carboxylic acid / type: ligand / ID: 2 / Number of copies: 1 / Formula: OJX |
|---|---|
| Molecular weight | Theoretical: 155.131 Da |
| Chemical component information | 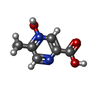 ChemComp-OJX: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 20 mM HEPES pH8.0, 100 mM NaCl, 1 mM MgCl2, 0.5 mM TCEP, 0.001% LMNG, 0.0001% CHS |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8k5c: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)