+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human full-length E6AP | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | Ubiquitin / LIGASE | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsperm entry / positive regulation of Golgi lumen acidification / regulation of ubiquitin-dependent protein catabolic process / prostate gland growth / HECT-type E3 ubiquitin transferase / androgen receptor signaling pathway / progesterone receptor signaling pathway / postsynaptic cytosol / protein K48-linked ubiquitination / protein autoubiquitination ...sperm entry / positive regulation of Golgi lumen acidification / regulation of ubiquitin-dependent protein catabolic process / prostate gland growth / HECT-type E3 ubiquitin transferase / androgen receptor signaling pathway / progesterone receptor signaling pathway / postsynaptic cytosol / protein K48-linked ubiquitination / protein autoubiquitination / ovarian follicle development / negative regulation of TORC1 signaling / proteasome complex / response to progesterone / positive regulation of protein ubiquitination / regulation of circadian rhythm / brain development / regulation of synaptic plasticity / protein polyubiquitination / ubiquitin-protein transferase activity / synaptic vesicle / ubiquitin protein ligase activity / rhythmic process / Antigen processing: Ubiquitination & Proteasome degradation / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / transcription coactivator activity / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / glutamatergic synapse / positive regulation of transcription by RNA polymerase II / proteolysis / zinc ion binding / nucleus / cytosol Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
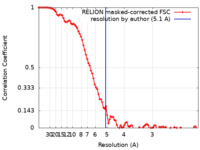
| Method | single particle reconstruction / cryo EM / Resolution: 5.1 Å | |||||||||||||||||||||
 Authors Authors | Wang Z / Yu X | |||||||||||||||||||||
| Funding support |  China, 6 items China, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural insights into the functional mechanism of the ubiquitin ligase E6AP. Authors: Zhen Wang / Fengying Fan / Zhihai Li / Fei Ye / Qingxia Wang / Rongchao Gao / Jiaxuan Qiu / Yixin Lv / Min Lin / Wenwen Xu / Cheng Luo / Xuekui Yu /  Abstract: E6AP dysfunction is associated with Angelman syndrome and Autism spectrum disorder. Additionally, the host E6AP is hijacked by the high-risk HPV E6 to aberrantly ubiquitinate the tumor suppressor ...E6AP dysfunction is associated with Angelman syndrome and Autism spectrum disorder. Additionally, the host E6AP is hijacked by the high-risk HPV E6 to aberrantly ubiquitinate the tumor suppressor p53, which is linked with development of multiple types of cancer, including most cervical cancers. Here we show that E6AP and the E6AP/E6 complex exist, respectively, as a monomer and a dimer of the E6AP/E6 protomer. The short α1-helix of E6AP transforms into a longer helical structure when in complex with E6. The extended α1-helices of the dimer intersect symmetrically and contribute to the dimerization. The two protomers sway around the crossed region of the two α1-helices to promote the attachment and detachment of substrates to the catalytic C-lobe of E6AP, thus facilitating ubiquitin transfer. These findings, complemented by mutagenesis analysis, suggest that the α1-helix, through conformational transformations, controls the transition between the inactive monomer and the active dimer of E6AP. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36604.map.gz emd_36604.map.gz | 59.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36604-v30.xml emd-36604-v30.xml emd-36604.xml emd-36604.xml | 14.9 KB 14.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36604_fsc.xml emd_36604_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_36604.png emd_36604.png | 22.9 KB | ||
| Filedesc metadata |  emd-36604.cif.gz emd-36604.cif.gz | 5.7 KB | ||
| Others |  emd_36604_half_map_1.map.gz emd_36604_half_map_1.map.gz emd_36604_half_map_2.map.gz emd_36604_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36604 http://ftp.pdbj.org/pub/emdb/structures/EMD-36604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36604 | HTTPS FTP |
-Related structure data
| Related structure data |  8jrs  8jrnC  8jroC  8jrpC  8jrqC  8jrrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36604.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36604.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36604_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36604_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E6AP
| Entire | Name: E6AP |
|---|---|
| Components |
|
-Supramolecule #1: E6AP
| Supramolecule | Name: E6AP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Ubiquitin-protein ligase E3A
| Macromolecule | Name: Ubiquitin-protein ligase E3A / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: HECT-type E3 ubiquitin transferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 100.811508 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEKLHQCYWK SGEPQSDDIE ASRMKRAAAK HLIERYYHQL TEGCGNEACT NEFCASCPTF LRMDNNAAAI KALELYKINA KLCDPHPSK KGASSAYLEN SKGAPNNSCS EIKMNKKGAR IDFKDVTYLT EEKVYEILEL CREREDYSPL IRVIGRVFSS A EALVQSFR ...String: MEKLHQCYWK SGEPQSDDIE ASRMKRAAAK HLIERYYHQL TEGCGNEACT NEFCASCPTF LRMDNNAAAI KALELYKINA KLCDPHPSK KGASSAYLEN SKGAPNNSCS EIKMNKKGAR IDFKDVTYLT EEKVYEILEL CREREDYSPL IRVIGRVFSS A EALVQSFR KVKQHTKEEL KSLQAKDEDK DEDEKEKAAC SAAAMEEDSE ASSSRIGDSS QGDNNLQKLG PDDVSVDIDA IR RVYTRLL SNEKIETAFL NALVYLSPNV ECDLTYHNVY SRDPNYLNLF IIVMENRNLH SPEYLEMALP LFCKAMSKLP LAA QGKLIR LWSKYNADQI RRMMETFQQL ITYKVISNEF NSRNLVNDDD AIVAASKCLK MVYYANVVGG EVDTNHNEED DEEP IPESS ELTLQELLGE ERRNKKGPRV DPLETELGVK TLDCRKPLIP FEEFINEPLN EVLEMDKDYT FFKVETENKF SFMTC PFIL NAVTKNLGLY YDNRIRMYSE RRITVLYSLV QGQQLNPYLR LKVRRDHIID DALVRLEMIA MENPADLKKQ LYVEFE GEQ GVDEGGVSKE FFQLVVEEIF NPDIGMFTYD ESTKLFWFNP SSFETEGQFT LIGIVLGLAI YNNCILDVHF PMVVYRK LM GKKGTFRDLG DSHPVLYQSL KDLLEYEGNV EDDMMITFQI SQTDLFGNPM MYDLKENGDK IPITNENRKE FVNLYSDY I LNKSVEKQFK AFRRGFHMVT NESPLKYLFR PEEIELLICG SRNLDFQALE ETTEYDGGYT RDSVLIREFW EIVHSFTDE QKRLFLQFTT GTDRAPVGGL GKLKMIIAKN GPDTERLPTS HTCFNVLLLP EYSSKEKLKE RLLKAITYAK GFGML UniProtKB: Ubiquitin-protein ligase E3A |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)