+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of PfAgo-guide DNA-target DNA complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nuclease / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationHydrolases; Acting on ester bonds; Site specific endodeoxyribonucleases: cleavage is not sequence specific (deleted sub-subclass) / clearance of foreign intracellular DNA / DNA endonuclease activity / manganese ion binding / DNA binding / RNA binding Similarity search - Function | |||||||||
| Biological species |   Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) / Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) /   Pyrococcus furiosus DSM 3638 (archaea) Pyrococcus furiosus DSM 3638 (archaea) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Zhuang L | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Molecular mechanism for target recognition, dimerization, and activation of Pyrococcus furiosus Argonaute. Authors: Longyu Wang / Wanping Chen / Chendi Zhang / Xiaochen Xie / Fuyong Huang / Miaomiao Chen / Wuxiang Mao / Na Yu / Qiang Wei / Lixin Ma / Zhuang Li /  Abstract: The Argonaute nuclease from the thermophilic archaeon Pyrococcus furiosus (PfAgo) contributes to host defense and represents a promising biotechnology tool. Here, we report the structure of a PfAgo- ...The Argonaute nuclease from the thermophilic archaeon Pyrococcus furiosus (PfAgo) contributes to host defense and represents a promising biotechnology tool. Here, we report the structure of a PfAgo-guide DNA-target DNA ternary complex at the cleavage-compatible state. The ternary complex is predominantly dimerized, and the dimerization is solely mediated by PfAgo at PIWI-MID, PIWI-PIWI, and PAZ-N interfaces. Additionally, PfAgo accommodates a short 14-bp guide-target DNA duplex with a wedge-type N domain and specifically recognizes 5'-phosphorylated guide DNA. In contrast, the PfAgo-guide DNA binary complex is monomeric, and the engagement of target DNA with 14-bp complementarity induces sufficient dimerization and activation of PfAgo, accompanied by movement of PAZ and N domains. A closely related Argonaute from Thermococcus thioreducens adopts a similar dimerization configuration with an additional zinc finger formed at the dimerization interface. Dimerization of both Argonautes stabilizes the catalytic loops, highlighting the important role of Argonaute dimerization in the activation and target cleavage. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36489.map.gz emd_36489.map.gz | 28.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36489-v30.xml emd-36489-v30.xml emd-36489.xml emd-36489.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
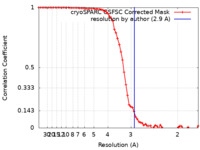
| FSC (resolution estimation) |  emd_36489_fsc.xml emd_36489_fsc.xml | 6.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_36489.png emd_36489.png | 98.8 KB | ||
| Filedesc metadata |  emd-36489.cif.gz emd-36489.cif.gz | 6.6 KB | ||
| Others |  emd_36489_half_map_1.map.gz emd_36489_half_map_1.map.gz emd_36489_half_map_2.map.gz emd_36489_half_map_2.map.gz | 28.2 MB 28.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36489 http://ftp.pdbj.org/pub/emdb/structures/EMD-36489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36489 | HTTPS FTP |
-Related structure data
| Related structure data |  8jpxMC  8wd8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36489.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36489.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
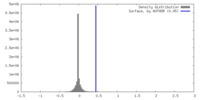
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36489_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
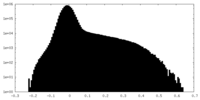
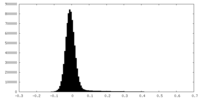
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36489_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
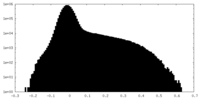
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of a PfAgo-guide DNA-target DNA complex
| Entire | Name: Cryo-EM structure of a PfAgo-guide DNA-target DNA complex |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of a PfAgo-guide DNA-target DNA complex
| Supramolecule | Name: Cryo-EM structure of a PfAgo-guide DNA-target DNA complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) |
-Macromolecule #1: Protein argonaute
| Macromolecule | Name: Protein argonaute / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Hydrolases; Acting on ester bonds; Site specific endodeoxyribonucleases: cleavage is not sequence specific (deleted sub-subclass) |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) Pyrococcus furiosus (strain ATCC 43587 / DSM 3638 / JCM 8422 / Vc1) (archaea) |
| Molecular weight | Theoretical: 90.510094 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKAIVVINLV KINKKIIPDK IYVYRLFNDP EEELQKEGYS IYRLAYENVG IVIDPENLII ATTKELEYEG EFIPEGEISF SELRNDYQS KLVLRLLKEN GIGEYELSKL LRKFRKPKTF GDYKVIPSVE MSVIKHDEDF YLVIHIIHQI QSMKTLWELV N KDPKELEE ...String: MKAIVVINLV KINKKIIPDK IYVYRLFNDP EEELQKEGYS IYRLAYENVG IVIDPENLII ATTKELEYEG EFIPEGEISF SELRNDYQS KLVLRLLKEN GIGEYELSKL LRKFRKPKTF GDYKVIPSVE MSVIKHDEDF YLVIHIIHQI QSMKTLWELV N KDPKELEE FLMTHKENLM LKDIASPLKT VYKPCFEEYT KKPKLDHNQE IVKYWYNYHI ERYWNTPEAK LEFYRKFGQV DL KQPAILA KFASKIKKNK NYKIYLLPQL VVPTYNAEQL ESDVAKEILE YTKLMPEERK ELLENILAEV DSDIIDKSLS EIE VEKIAQ ELENKIRVRD DKGNSVPISQ LNVQKSQLLL WTNYSRKYPV ILPYEVPEKF RKIREIPMFI ILDSGLLADI QNFA TNEFR ELVKSMYYSL AKKYNSLAKK ARSTNEIGLP FLDFRGKEKV ITEDLNSDKG IIEVVEQVSS FMKGKELGLA FIAAR NKLS SEKFEEIKRR LFNLNVISQV VNEDTLKNKR DKYDRNRLDL FVRHNLLFQV LSKLGVKYYV LDYRFNYDYI IGIDVA PMK RSEGYIGGSA VMFDSQGYIR KIVPIKIGEQ RGESVDMNEF FKEMVDKFKE FNIKLDNKKI LLLRDGRITN NEEEGLK YI SEMFDIEVVT MDVIKNHPVR AFANMKMYFN LGGAIYLIPH KLKQAKGTPI PIKLAKKRII KNGKVEKQSI TRQDVLDI F ILTRLNYGSI SADMRLPAPV HYAHKFANAI RNEWKIKEEF LAEGFLYFV UniProtKB: Protein argonaute |
-Macromolecule #2: Guide DNA
| Macromolecule | Name: Guide DNA / type: dna / ID: 2 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus DSM 3638 (archaea) Pyrococcus furiosus DSM 3638 (archaea) |
| Molecular weight | Theoretical: 5.337467 KDa |
| Sequence | String: (DT)(DG)(DA)(DG)(DG)(DT)(DA)(DG)(DT)(DA) (DG)(DG)(DT)(DT)(DG)(DT)(DA) |
-Macromolecule #3: Target DNA
| Macromolecule | Name: Target DNA / type: dna / ID: 3 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus DSM 3638 (archaea) Pyrococcus furiosus DSM 3638 (archaea) |
| Molecular weight | Theoretical: 5.075327 KDa |
| Sequence | String: (DA)(DC)(DA)(DA)(DC)(DC)(DT)(DA)(DC)(DT) (DA)(DC)(DC)(DT)(DC)(DA)(DT) |
-Macromolecule #4: Excess DNA
| Macromolecule | Name: Excess DNA / type: dna / ID: 4 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus DSM 3638 (archaea) Pyrococcus furiosus DSM 3638 (archaea) |
| Molecular weight | Theoretical: 1.780199 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT) |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 6 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)