+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | membrane proteins | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | enzyme / acetylation / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationheparan-alpha-glucosaminide N-acetyltransferase / heparan-alpha-glucosaminide N-acetyltransferase activity / MPS IIIC - Sanfilippo syndrome C / heparan sulfate proteoglycan catabolic process / HS-GAG degradation / lysosomal transport / acyltransferase activity / protein complex oligomerization / tertiary granule membrane / specific granule membrane ...heparan-alpha-glucosaminide N-acetyltransferase / heparan-alpha-glucosaminide N-acetyltransferase activity / MPS IIIC - Sanfilippo syndrome C / heparan sulfate proteoglycan catabolic process / HS-GAG degradation / lysosomal transport / acyltransferase activity / protein complex oligomerization / tertiary granule membrane / specific granule membrane / lysosomal lumen / lysosomal membrane / Neutrophil degranulation / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
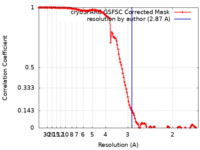
| Method | single particle reconstruction / cryo EM / Resolution: 2.87 Å | |||||||||
 Authors Authors | Yu J / Ge JP / Ruisheng X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structure and mechanism of lysosome transmembrane acetylation by HGSNAT. Authors: Ruisheng Xu / Yingjie Ning / Fandong Ren / Chenxia Gu / Zhengjiang Zhu / Xuefang Pan / Alexey V Pshezhetsky / Jingpeng Ge / Jie Yu /   Abstract: Lysosomal transmembrane acetylation of heparan sulfates (HS) is catalyzed by HS acetyl-CoA:α-glucosaminide N-acetyltransferase (HGSNAT), whose dysfunction leads to lysosomal storage diseases. The ...Lysosomal transmembrane acetylation of heparan sulfates (HS) is catalyzed by HS acetyl-CoA:α-glucosaminide N-acetyltransferase (HGSNAT), whose dysfunction leads to lysosomal storage diseases. The mechanism by which HGSNAT, the sole non-hydrolase enzyme in HS degradation, brings cytosolic acetyl-coenzyme A (Ac-CoA) and lysosomal HS together for N-acyltransferase reactions remains unclear. Here, we present cryogenic-electron microscopy structures of HGSNAT alone, complexed with Ac-CoA and with acetylated products. These structures explain that Ac-CoA binding from the cytosolic side causes dimeric HGSNAT to form a transmembrane tunnel. Within this tunnel, catalytic histidine and asparagine approach the lumen and instigate the transfer of the acetyl group from Ac-CoA to the glucosamine group of HS. Our study unveils a transmembrane acetylation mechanism that may help advance therapeutic strategies targeting lysosomal storage diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36376.map.gz emd_36376.map.gz | 229.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36376-v30.xml emd-36376-v30.xml emd-36376.xml emd-36376.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36376_fsc.xml emd_36376_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_36376.png emd_36376.png | 92.7 KB | ||
| Filedesc metadata |  emd-36376.cif.gz emd-36376.cif.gz | 6.7 KB | ||
| Others |  emd_36376_half_map_1.map.gz emd_36376_half_map_1.map.gz emd_36376_half_map_2.map.gz emd_36376_half_map_2.map.gz | 226.5 MB 226.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36376 http://ftp.pdbj.org/pub/emdb/structures/EMD-36376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36376 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36376 | HTTPS FTP |
-Validation report
| Summary document |  emd_36376_validation.pdf.gz emd_36376_validation.pdf.gz | 765.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36376_full_validation.pdf.gz emd_36376_full_validation.pdf.gz | 764.8 KB | Display | |
| Data in XML |  emd_36376_validation.xml.gz emd_36376_validation.xml.gz | 21.6 KB | Display | |
| Data in CIF |  emd_36376_validation.cif.gz emd_36376_validation.cif.gz | 27.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36376 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36376 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36376 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36376 | HTTPS FTP |
-Related structure data
| Related structure data |  8jkvMC  8jl1C  8jl3C  8w4aC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36376.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36376.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36376_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36376_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : HG
| Entire | Name: HG |
|---|---|
| Components |
|
-Supramolecule #1: HG
| Supramolecule | Name: HG / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Heparan-alpha-glucosaminide N-acetyltransferase
| Macromolecule | Name: Heparan-alpha-glucosaminide N-acetyltransferase / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: heparan-alpha-glucosaminide N-acetyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 77.183992 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MTGARASAAE QRRAGRSGQA RAAERAAGMS GAGRALAALL LAASVLSAAL LAPGGSSGRD AQAAPPRDLD KKRHAELKMD QALLLIHNE LLWTNLTVYW KSECCYHCLF QVLVNVPQSP KAGKPSAAAA SVSTQHGSIL QLNDTLEEKE VCRLEYRFGE F GNYSLLVK ...String: MTGARASAAE QRRAGRSGQA RAAERAAGMS GAGRALAALL LAASVLSAAL LAPGGSSGRD AQAAPPRDLD KKRHAELKMD QALLLIHNE LLWTNLTVYW KSECCYHCLF QVLVNVPQSP KAGKPSAAAA SVSTQHGSIL QLNDTLEEKE VCRLEYRFGE F GNYSLLVK NIHNGVSEIA CDLAVNEDPV DSNLPVSIAF LIGLAVIIVI SFLRLLLSLD DFNNWISKAI SSRETDRLIN SE LGSPSRT DPLDGDVQPA TWRLSALPPR LRSVDTFRGI ALILMVFVNY GGGKYWYFKH ASWNGLTVAD LVFPWFVFIM GSS IFLSMT SILQRGCSKF RLLGKIAWRS FLLICIGIII VNPNYCLGPL SWDKVRIPGV LQRLGVTYFV VAVLELLFAK PVPE HCASE RSCLSLRDIT SSWPQWLLIL VLEGLWLGLT FLLPVPGCPT GYLGPGGIGD FGKYPNCTGG AAGYIDRLLL GDDHL YQHP SSAVLYHTEV AYDPEGILGT INSIVMAFLG VQAGKILLYY KARTKDILIR FTAWCCILGL ISVALTKVSE NEGFIP VNK NLWSLSYVTT LSSFAFFILL VLYPVVDVKG LWTGTPFFYP GMNSILVYVG HEVFENYFPF QWKLKDNQSH KEHLTQN IV ATALWVLIAY ILYRKKIFWK IGSGGGGSGG GGSGGGWSHP QFEKGGGSGG GSGGSAWSHP QFEK UniProtKB: Heparan-alpha-glucosaminide N-acetyltransferase |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 2 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: DODECANE
| Macromolecule | Name: DODECANE / type: ligand / ID: 4 / Number of copies: 6 / Formula: D12 |
|---|---|
| Molecular weight | Theoretical: 170.335 Da |
| Chemical component information |  ChemComp-D12: |
-Macromolecule #5: N-OCTANE
| Macromolecule | Name: N-OCTANE / type: ligand / ID: 5 / Number of copies: 30 / Formula: OCT |
|---|---|
| Molecular weight | Theoretical: 114.229 Da |
| Chemical component information |  ChemComp-OCT: |
-Macromolecule #6: TETRADECANE
| Macromolecule | Name: TETRADECANE / type: ligand / ID: 6 / Number of copies: 24 / Formula: C14 |
|---|---|
| Molecular weight | Theoretical: 198.388 Da |
| Chemical component information |  ChemComp-C14: |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 2 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #8: DECANE
| Macromolecule | Name: DECANE / type: ligand / ID: 8 / Number of copies: 2 / Formula: D10 |
|---|---|
| Molecular weight | Theoretical: 142.282 Da |
| Chemical component information |  ChemComp-D10: |
-Macromolecule #9: HEXADECANE
| Macromolecule | Name: HEXADECANE / type: ligand / ID: 9 / Number of copies: 2 / Formula: R16 |
|---|---|
| Molecular weight | Theoretical: 226.441 Da |
| Chemical component information |  ChemComp-R16: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)