[English] 日本語
 Yorodumi
Yorodumi- EMDB-36367: Cryo-EM structure of KCTD5 in complex with Gbeta gamma subunits -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of KCTD5 in complex with Gbeta gamma subunits | |||||||||
 Map data Map data | EM density map of KCTD5_Gng2/Gnb1 complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ubiquitination / GPCR signaling / PROTEIN BINDING | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein homooligomerization / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation ...protein homooligomerization / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / G protein-coupled receptor signaling pathway / lysosomal membrane / GTPase activity / synapse / protein-containing complex binding / signal transduction / extracellular exosome / nucleus / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.27 Å | |||||||||
 Authors Authors | Zheng S / Jiang W / Wang W / Kong Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Structural basis for the ubiquitination of G protein βγ subunits by KCTD5/Cullin3 E3 ligase. Authors: Wentong Jiang / Wei Wang / Yinfei Kong / Sanduo Zheng /  Abstract: G protein-coupled receptor (GPCR) signaling is precisely controlled to avoid overstimulation that results in detrimental consequences. Gβγ signaling is negatively regulated by a Cullin3 (Cul3)- ...G protein-coupled receptor (GPCR) signaling is precisely controlled to avoid overstimulation that results in detrimental consequences. Gβγ signaling is negatively regulated by a Cullin3 (Cul3)-dependent E3 ligase, KCTD5, which triggers ubiquitination and degradation of free Gβγ. Here, we report the cryo-electron microscopy structures of the KCTD5-Gβγ fusion complex and the KCTD7-Cul3 complex. KCTD5 in pentameric form engages symmetrically with five copies of Gβγ through its C-terminal domain. The unique pentameric assembly of the KCTD5/Cul3 E3 ligase places the ubiquitin-conjugating enzyme (E2) and the modification sites of Gβγ in close proximity and allows simultaneous transfer of ubiquitin from E2 to five Gβγ subunits. Moreover, we show that ubiquitination of Gβγ by KCTD5 is important for fine-tuning cyclic adenosine 3´,5´-monophosphate signaling of GPCRs. Our studies provide unprecedented insights into mechanisms of substrate recognition by unusual pentameric E3 ligases and highlight the KCTD family as emerging regulators of GPCR signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36367.map.gz emd_36367.map.gz | 80.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36367-v30.xml emd-36367-v30.xml emd-36367.xml emd-36367.xml | 23.3 KB 23.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36367.png emd_36367.png | 58 KB | ||
| Filedesc metadata |  emd-36367.cif.gz emd-36367.cif.gz | 6.8 KB | ||
| Others |  emd_36367_additional_1.map.gz emd_36367_additional_1.map.gz emd_36367_half_map_1.map.gz emd_36367_half_map_1.map.gz emd_36367_half_map_2.map.gz emd_36367_half_map_2.map.gz | 154 MB 151.6 MB 151.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36367 http://ftp.pdbj.org/pub/emdb/structures/EMD-36367 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36367 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36367 | HTTPS FTP |
-Validation report
| Summary document |  emd_36367_validation.pdf.gz emd_36367_validation.pdf.gz | 694.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36367_full_validation.pdf.gz emd_36367_full_validation.pdf.gz | 693.9 KB | Display | |
| Data in XML |  emd_36367_validation.xml.gz emd_36367_validation.xml.gz | 14.6 KB | Display | |
| Data in CIF |  emd_36367_validation.cif.gz emd_36367_validation.cif.gz | 17.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36367 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36367 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36367 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36367 | HTTPS FTP |
-Related structure data
| Related structure data |  8jkbMC  8i79C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36367.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36367.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM density map of KCTD5_Gng2/Gnb1 complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
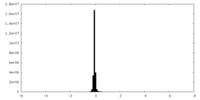
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: EM density map of KCTD5 Gng2/Gnb1 complex after sharpening
| File | emd_36367_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM density map of KCTD5_Gng2/Gnb1 complex after sharpening | ||||||||||||
| Projections & Slices |
| ||||||||||||
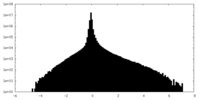
| Density Histograms |
-Half map: half map of KCTD5 Gng2/Gnb1 complex
| File | emd_36367_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map of KCTD5_Gng2/Gnb1 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
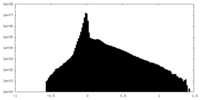
| Density Histograms |
-Half map: half map of KCTD5 Gng2/Gnb1 complex
| File | emd_36367_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map of KCTD5_Gng2/Gnb1 complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of KCTD5 in complex with G protein betagamma su...
| Entire | Name: Cryo-EM structure of KCTD5 in complex with G protein betagamma subunits |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of KCTD5 in complex with G protein betagamma su...
| Supramolecule | Name: Cryo-EM structure of KCTD5 in complex with G protein betagamma subunits type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.088762 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MYPYDVPDYA YPYDVPDYAY PYDVPDYAGS GSMSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAK IYAMHWGTDS RLLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK T REGNVRVS ...String: MYPYDVPDYA YPYDVPDYAY PYDVPDYAGS GSMSELDQLR QEAEQLKNQI RDARKACADA TLSQITNNID PVGRIQMRTR RTLRGHLAK IYAMHWGTDS RLLVSASQDG KLIIWDSYTT NKVHAIPLRS SWVMTCAYAP SGNYVACGGL DNICSIYNLK T REGNVRVS RELAGHTGYL SCCRFLDDNQ IVTSSGDTTC ALWDIETGQQ TTTFTGHTGD VMSLSLAPDT RLFVSGACDA SA KLWDVRE GMCRQTFTGH ESDINAICFF PNGNAFATGS DDATCRLFDL RADQELMTYS HDNIICGITS VSFSKSGRLL LAG YDDFNC NVWDALKADR AGVLAGHDNR VSCLGVTDDG MAVATGSWDS FLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 2 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.228731 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSALEVLFQG PGAGALEVLF QGPGSMASNN TASIAQARKL VEQLKMEANI DRIKVSKAAA DLMAYCEAHA KEDPLLTPVP ASENPFREK KFFSAIL UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #3: BTB/POZ domain-containing protein KCTD5
| Macromolecule | Name: BTB/POZ domain-containing protein KCTD5 / type: protein_or_peptide / ID: 3 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.780623 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGSSHHHHHH GSEDQVDPRL IDGKDYKDDD DKLEVLFQGP MAENHCELLP PAPSGLGAGL GGGLCRRCSA GMGALAQRPG GVSKWVRLN VGGTYFLTTR QTLCRDPKSF LYRLCQADPD LDSDKDETGA YLIDRDPTYF GPVLNYLRHG KLVINKDLAE E GVLEEAEF ...String: MGSSHHHHHH GSEDQVDPRL IDGKDYKDDD DKLEVLFQGP MAENHCELLP PAPSGLGAGL GGGLCRRCSA GMGALAQRPG GVSKWVRLN VGGTYFLTTR QTLCRDPKSF LYRLCQADPD LDSDKDETGA YLIDRDPTYF GPVLNYLRHG KLVINKDLAE E GVLEEAEF YNITSLIKLV KDKIRERDSR ISQMPVKHVY RVLQCQEEEL TQMVSTMSDG WKFEQLVSIG SSYNYGNEDQ AE FLCVVSK ELHNTPYGTT SEPSEKAKIL QERGSRM UniProtKB: BTB/POZ domain-containing protein KCTD5 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.32 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 49.71 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)