+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of apo state mClC-3_I607T | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationvolume-sensitive chloride channel activity / chloride:proton antiporter activity / negative regulation of cell volume / synaptic vesicle lumen acidification / inhibitory synapse / specific granule / voltage-gated chloride channel activity / photoreceptor cell maintenance / vesicle membrane / antiporter activity ...volume-sensitive chloride channel activity / chloride:proton antiporter activity / negative regulation of cell volume / synaptic vesicle lumen acidification / inhibitory synapse / specific granule / voltage-gated chloride channel activity / photoreceptor cell maintenance / vesicle membrane / antiporter activity / phagocytosis, engulfment / synaptic transmission, GABAergic / positive regulation of reactive oxygen species biosynthetic process / phagocytic vesicle / axon terminus / adult locomotory behavior / PDZ domain binding / synaptic transmission, glutamatergic / recycling endosome / neuron cellular homeostasis / GABA-ergic synapse / recycling endosome membrane / synaptic vesicle / synaptic vesicle membrane / late endosome membrane / late endosome / early endosome membrane / early endosome / endosome / endosome membrane / lysosomal membrane / external side of plasma membrane / glutamatergic synapse / Golgi apparatus / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.96 Å | |||||||||
 Authors Authors | Wan YZQ / Yang F | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis of adenine nucleotides regulation and neurodegenerative pathology in ClC-3 exchanger. Authors: Yangzhuoqun Wan / Shuangshuang Guo / Wenxuan Zhen / Lizhen Xu / Xiaoying Chen / Fangyue Liu / Yi Shen / Shuangshuang Liu / Lidan Hu / Xinyan Wang / Fengcan Ye / Qinrui Wang / Han Wen / Fan Yang /  Abstract: The ClC-3 chloride/proton exchanger is both physiologically and pathologically critical, as it is potentiated by ATP to detect metabolic energy level and point mutations in ClC-3 lead to severe ...The ClC-3 chloride/proton exchanger is both physiologically and pathologically critical, as it is potentiated by ATP to detect metabolic energy level and point mutations in ClC-3 lead to severe neurodegenerative diseases in human. However, why this exchanger is differentially modulated by ATP, ADP or AMP and how mutations caused gain-of-function remains largely unknow. Here we determine the high-resolution structures of dimeric wildtype ClC-3 in the apo state and in complex with ATP, ADP and AMP, and the disease-causing I607T mutant in the apo and ATP-bounded state by cryo-electron microscopy. In combination with patch-clamp recordings and molecular dynamic simulations, we reveal how the adenine nucleotides binds to ClC-3 and changes in ion occupancy between apo and ATP-bounded state. We further observe I607T mutation induced conformational changes and augments in current. Therefore, our study not only lays the structural basis of adenine nucleotides regulation in ClC-3, but also clearly indicates the target region for drug discovery against ClC-3 mediated neurodegenerative diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36245.map.gz emd_36245.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36245-v30.xml emd-36245-v30.xml emd-36245.xml emd-36245.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36245.png emd_36245.png | 24.6 KB | ||
| Filedesc metadata |  emd-36245.cif.gz emd-36245.cif.gz | 6.2 KB | ||
| Others |  emd_36245_half_map_1.map.gz emd_36245_half_map_1.map.gz emd_36245_half_map_2.map.gz emd_36245_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36245 http://ftp.pdbj.org/pub/emdb/structures/EMD-36245 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36245 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36245 | HTTPS FTP |
-Validation report
| Summary document |  emd_36245_validation.pdf.gz emd_36245_validation.pdf.gz | 937.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_36245_full_validation.pdf.gz emd_36245_full_validation.pdf.gz | 937.2 KB | Display | |
| Data in XML |  emd_36245_validation.xml.gz emd_36245_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_36245_validation.cif.gz emd_36245_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36245 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36245 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36245 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-36245 | HTTPS FTP |
-Related structure data
| Related structure data |  8jgsMC  8jevC  8jgjC  8jgkC  8jglC  8jgvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36245.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36245.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
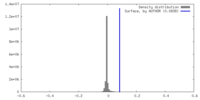
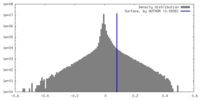
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : apo state of mClC-3_I607T
| Entire | Name: apo state of mClC-3_I607T |
|---|---|
| Components |
|
-Supramolecule #1: apo state of mClC-3_I607T
| Supramolecule | Name: apo state of mClC-3_I607T / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: H(+)/Cl(-) exchange transporter 3
| Macromolecule | Name: H(+)/Cl(-) exchange transporter 3 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 90.945766 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MESEQLFHRG YYRNSYNSIT SASSDEELLD GAGAIMDFQT SEDDNLLDGD TAAGTHYTMT NGGSINSSTH LLDLLDEPIP GVGTYDDFH TIDWVREKCK DRERHRRINS KKKESAWEMT KSLYDAWSGW LVVTLTGLAS GALAGLIDIA ADWMTDLKEG I CLSALWYN ...String: MESEQLFHRG YYRNSYNSIT SASSDEELLD GAGAIMDFQT SEDDNLLDGD TAAGTHYTMT NGGSINSSTH LLDLLDEPIP GVGTYDDFH TIDWVREKCK DRERHRRINS KKKESAWEMT KSLYDAWSGW LVVTLTGLAS GALAGLIDIA ADWMTDLKEG I CLSALWYN HEQCCWGSNE TTFEERDKCP QWKTWAELII GQAEGPGSYI MNYIMYIFWA LSFAFLAVSL VKVFAPYACG SG IPEIKTI LSGFIIRGYL GKWTLMIKTI TLVLAVASGL SLGKEGPLVH VACCCGNIFS YLFPKYSTNE AKKREVLSAA SAA GVSVAF GAPIGGVLFS LEEVSYYFPL KTLWRSFFAA LVAAFVLRSI NPFGNSRLVL FYVEYHTPWY LFELFPFILL GVFG GLWGA FFIRANIAWC RRRKSTKFGK YPVLEVIIVA AITAVIAFPN PYTRLNTSEL IKELFTDCGP LESSSLCDYR NDMNA SKIV DDIPDRPAGV GVYSAIWQLC LALIFKIIMT VFTFGIKVPS GLFIPSMAIG AIAGRIVGIA VEQLAYYHHD WFIFKE WCE VGADCITPGL YAMVGAAACL GGVTRMTVSL VVIVFELTGG LEYTVPLMAA VMTSKWVGDA FGREGIYEAH IRLNGYP FL DAKEEFTHTT LAADVMRPRR SDPPLAVLTQ DNMTVDDIEN MINETSYNGF PVIMSKESQR LVGFALRRDL TIAIESAR K KQEGIVGSSR VCFAQHTPSL PAESPRPLKL RSILDMSPFT VTDHTPMEIV VDIFRKLGLR QCLVTHNGRL LGIITKKDI LRHMAQTANQ DPASIMFN UniProtKB: H(+)/Cl(-) exchange transporter 3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)