[English] 日本語
 Yorodumi
Yorodumi- EMDB-35966: Cryo-EM structure of SARS-CoV-2 BA.4/5 spike protein in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 BA.4/5 spike protein in complex with 1G11 (state 1) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Neutralizing antibody / Cryo-EM / VIRAL PROTEIN / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.73 Å | |||||||||
 Authors Authors | Sun H / Jiang Y / Zheng Z / Zheng Q / Li S | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: J Virol / Year: 2023 Journal: J Virol / Year: 2023Title: Structural basis for broad neutralization of human antibody against Omicron sublineages and evasion by XBB variant. Authors: Hui Sun / Yizhen Wang / Xiuting Chen / Yanan Jiang / Siling Wang / Yang Huang / Liqin Liu / Yu Li / Miaolin Lan / Huilin Guo / Quan Yuan / Yali Zhang / Tingting Li / Hai Yu / Ying Gu / Jun ...Authors: Hui Sun / Yizhen Wang / Xiuting Chen / Yanan Jiang / Siling Wang / Yang Huang / Liqin Liu / Yu Li / Miaolin Lan / Huilin Guo / Quan Yuan / Yali Zhang / Tingting Li / Hai Yu / Ying Gu / Jun Zhang / Shaowei Li / Zizheng Zheng / Qingbing Zheng / Ningshao Xia /  Abstract: The ongoing COVID-19 pandemic has been characterized by the emergence of new SARS-CoV-2 variants including the highly transmissible Omicron XBB sublineages, which have shown significant resistance to ...The ongoing COVID-19 pandemic has been characterized by the emergence of new SARS-CoV-2 variants including the highly transmissible Omicron XBB sublineages, which have shown significant resistance to neutralizing antibodies (nAbs). This resistance has led to decreased vaccine effectiveness and therefore result in breakthrough infections and reinfections, which continuously threaten public health. To date, almost all available therapeutic nAbs, including those authorized under Emergency Use Authorization nAbs that were previously clinically useful against early strains, have recently been found to be ineffective against newly emerging variants. In this study, we provide a comprehensive structural basis about how the Class 3 nAbs, including 1G11 in this study and noted LY-CoV1404, are evaded by the newly emerged SARS-CoV-2 variants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35966.map.gz emd_35966.map.gz | 361.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35966-v30.xml emd-35966-v30.xml emd-35966.xml emd-35966.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35966.png emd_35966.png | 31.1 KB | ||
| Filedesc metadata |  emd-35966.cif.gz emd-35966.cif.gz | 3.9 KB | ||
| Others |  emd_35966_half_map_1.map.gz emd_35966_half_map_1.map.gz emd_35966_half_map_2.map.gz emd_35966_half_map_2.map.gz | 676.7 MB 676.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35966 http://ftp.pdbj.org/pub/emdb/structures/EMD-35966 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35966 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35966 | HTTPS FTP |
-Validation report
| Summary document |  emd_35966_validation.pdf.gz emd_35966_validation.pdf.gz | 969.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35966_full_validation.pdf.gz emd_35966_full_validation.pdf.gz | 969.1 KB | Display | |
| Data in XML |  emd_35966_validation.xml.gz emd_35966_validation.xml.gz | 20.5 KB | Display | |
| Data in CIF |  emd_35966_validation.cif.gz emd_35966_validation.cif.gz | 24.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35966 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35966 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35966 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35966 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35966.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35966.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.778 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_35966_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
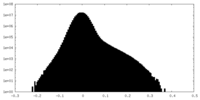
| Projections & Slices |
| ||||||||||||
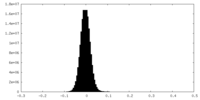
| Density Histograms |
-Half map: #2
| File | emd_35966_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
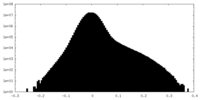
| Projections & Slices |
| ||||||||||||
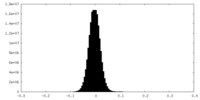
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 BA.4/5 spike protein in complex with 1G11
| Entire | Name: SARS-CoV-2 BA.4/5 spike protein in complex with 1G11 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 BA.4/5 spike protein in complex with 1G11
| Supramolecule | Name: SARS-CoV-2 BA.4/5 spike protein in complex with 1G11 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: SARS-CoV-2 BA.4/5 spike protein
| Supramolecule | Name: SARS-CoV-2 BA.4/5 spike protein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: nAb 1G11
| Supramolecule | Name: nAb 1G11 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.73 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 275583 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X