+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GMPCPP-Alpha4A/Beta2A-microtubule decorated with kinesin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | microtubule / tubulin isotype / cryo-EM structure / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMicrotubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III / Carboxyterminal post-translational modifications of tubulin / Intraflagellar transport / COPI-independent Golgi-to-ER retrograde traffic / regulation of modification of synapse structure, modulating synaptic transmission / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / plus-end-directed vesicle transport along microtubule / cytoplasm organization ...Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III / Carboxyterminal post-translational modifications of tubulin / Intraflagellar transport / COPI-independent Golgi-to-ER retrograde traffic / regulation of modification of synapse structure, modulating synaptic transmission / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / plus-end-directed vesicle transport along microtubule / cytoplasm organization / cytolytic granule membrane / anterograde dendritic transport of neurotransmitter receptor complex / COPI-mediated anterograde transport / Aggrephagy / Kinesins / anterograde neuronal dense core vesicle transport / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Resolution of Sister Chromatid Cohesion / PKR-mediated signaling / mitocytosis / retrograde neuronal dense core vesicle transport / The role of GTSE1 in G2/M progression after G2 checkpoint / RHO GTPases activate IQGAPs / Recycling pathway of L1 / anterograde axonal protein transport / COPI-dependent Golgi-to-ER retrograde traffic / RHO GTPases Activate Formins / Separation of Sister Chromatids / Hedgehog 'off' state / Loss of Nlp from mitotic centrosomes / Recruitment of mitotic centrosome proteins and complexes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / AURKA Activation by TPX2 / Platelet degranulation / Regulation of PLK1 Activity at G2/M Transition / ciliary rootlet / lysosome localization / positive regulation of potassium ion transport / MHC class II antigen presentation / plus-end-directed microtubule motor activity / vesicle transport along microtubule / Kinesins / RHO GTPases activate KTN1 / kinesin complex / microtubule motor activity / centrosome localization / mitochondrion transport along microtubule / COPI-dependent Golgi-to-ER retrograde traffic / microtubule-based movement / stress granule disassembly / natural killer cell mediated cytotoxicity / Insulin processing / synaptic vesicle transport / postsynaptic cytosol / microtubule-based process / intercellular bridge / phagocytic vesicle / axon cytoplasm / MHC class II antigen presentation / dendrite cytoplasm / axon guidance / positive regulation of synaptic transmission, GABAergic / regulation of membrane potential / positive regulation of protein localization to plasma membrane / cerebral cortex development / structural constituent of cytoskeleton / cellular response to type II interferon / centriolar satellite / mitotic spindle / Signaling by ALK fusions and activated point mutants / nuclear membrane / microtubule binding / vesicle / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / microtubule / cilium / hydrolase activity / cadherin binding / GTPase activity / protein kinase binding / GTP binding / protein-containing complex binding / perinuclear region of cytoplasm / enzyme binding / ATP hydrolysis activity / mitochondrion / ATP binding / metal ion binding / identical protein binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Zheng W / Zhao QY / Diao L / Bao L / Cong Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Acta Biochim Biophys Sin (Shanghai) / Year: 2023 Journal: Acta Biochim Biophys Sin (Shanghai) / Year: 2023Title: Cryo-EM of α-tubulin isotype-containing microtubules revealed a contracted structure of α4A/β2A microtubules. Authors: Lei Diao / Wei Zheng / Qiaoyu Zhao / Mingyi Liu / Zhenglin Fu / Xu Zhang / Lan Bao / Yao Cong /  Abstract: Microtubules are hollow α/β-tubulin heterodimeric polymers that play critical roles in cells. In vertebrates, both α- and β-tubulins have multiple isotypes encoded by different genes, which are ...Microtubules are hollow α/β-tubulin heterodimeric polymers that play critical roles in cells. In vertebrates, both α- and β-tubulins have multiple isotypes encoded by different genes, which are intrinsic factors in regulating microtubule functions. However, the structures of microtubules composed of different tubulin isotypes, especially α-tubulin isotypes, remain largely unknown. Here, we purify recombinant tubulin heterodimers composed of different mouse α-tubulin isotypes, including α1A, α1C and α4A, with the β-tubulin isotype β2A. We further assemble and determine the cryo-electron microscopy (cryo-EM) structures of α1A/β2A, α1C/β2A, and α4A/β2A microtubules. Our structural analysis demonstrates that α4A/β2A microtubules exhibit longitudinal contraction between tubulin interdimers compared with α1A/β2A and α1C/β2A microtubules. Collectively, our findings reveal that α-tubulin isotype composition can tune microtubule structures, and also provide evidence for the "tubulin code" hypothesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35792.map.gz emd_35792.map.gz | 458.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35792-v30.xml emd-35792-v30.xml emd-35792.xml emd-35792.xml | 17.9 KB 17.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35792.png emd_35792.png | 73.3 KB | ||
| Filedesc metadata |  emd-35792.cif.gz emd-35792.cif.gz | 6.6 KB | ||
| Others |  emd_35792_half_map_1.map.gz emd_35792_half_map_1.map.gz emd_35792_half_map_2.map.gz emd_35792_half_map_2.map.gz | 146.5 MB 146.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35792 http://ftp.pdbj.org/pub/emdb/structures/EMD-35792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35792 | HTTPS FTP |
-Validation report
| Summary document |  emd_35792_validation.pdf.gz emd_35792_validation.pdf.gz | 887.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35792_full_validation.pdf.gz emd_35792_full_validation.pdf.gz | 886.7 KB | Display | |
| Data in XML |  emd_35792_validation.xml.gz emd_35792_validation.xml.gz | 18.7 KB | Display | |
| Data in CIF |  emd_35792_validation.cif.gz emd_35792_validation.cif.gz | 22.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35792 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35792 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35792 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35792 | HTTPS FTP |
-Related structure data
| Related structure data |  8ixfMC  8ixgMC  8ixaC  8ixbC  8ixdC  8ixeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35792.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35792.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.318 Å | ||||||||||||||||||||||||||||||||||||
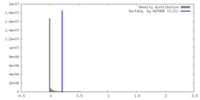
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35792_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
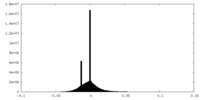
| Density Histograms |
-Half map: #1
| File | emd_35792_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
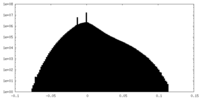
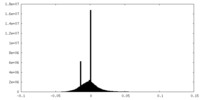
| Density Histograms |
- Sample components
Sample components
-Entire : GMPCPP-Alpha4A/Beta2A-microtubule decorated with kinesin
| Entire | Name: GMPCPP-Alpha4A/Beta2A-microtubule decorated with kinesin |
|---|---|
| Components |
|
-Supramolecule #1: GMPCPP-Alpha4A/Beta2A-microtubule decorated with kinesin
| Supramolecule | Name: GMPCPP-Alpha4A/Beta2A-microtubule decorated with kinesin type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Tubulin alpha-4A chain
| Macromolecule | Name: Tubulin alpha-4A chain / type: protein_or_peptide / ID: 1 Details: Author stated: the data processing is a non-standard procedure for microtubule-type of helical reconstruction with a seam, and it was performed around 2017 to 2019. We followed the procedure ...Details: Author stated: the data processing is a non-standard procedure for microtubule-type of helical reconstruction with a seam, and it was performed around 2017 to 2019. We followed the procedure developed by Dr. Rui Zhang (R. Zhang, Cell, 2015, doi: 10.1016/j.cell.2015.07.012), with all the scripts provided by him. In this way, the generated half-maps have an applied circular mask but without touching the structural outer boundaries. Number of copies: 9 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 50.806109 KDa |
| Recombinant expression | Organism:  Insecta environmental sample (insect) Insecta environmental sample (insect) |
| Sequence | String: MRECISVHVG QAGVQMGNAC WELYCLEHGI QPDGQMPSDK TIHHHHHHGG GDDSFTTFFC ETGAGKHVPR AVFVDLEPTV IDEIRNGPY RQLFHPEQLI TGKEDAANNY ARGHYTIGKE IIDPVLDRIR KLSDQCTGLQ GFLVFHSFGG GTGSGFTSLL M ERLSVDYG ...String: MRECISVHVG QAGVQMGNAC WELYCLEHGI QPDGQMPSDK TIHHHHHHGG GDDSFTTFFC ETGAGKHVPR AVFVDLEPTV IDEIRNGPY RQLFHPEQLI TGKEDAANNY ARGHYTIGKE IIDPVLDRIR KLSDQCTGLQ GFLVFHSFGG GTGSGFTSLL M ERLSVDYG KKSKLEFSIY PAPQVSTAVV EPYNSILTTH TTLEHSDCAF MVDNEAIYDI CRRNLDIERP TYTNLNRLIS QI VSSITAS LRFDGALNVD LTEFQTNLVP YPRIHFPLAT YAPVISAEKA YHEQLSVAEI TNACFEPANQ MVKCDPRHGK YMA CCLLYR GDVVPKDVNA AIAAIKTKRS IQFVDWCPTG FKVGINYQPP TVVPGGDLAK VQRAVCMLSN TTAIAEAWAR LDHK FDLMY AKRAFVHWYV GEGMEEGEFS EAREDMAALE KDYEEVGIDS YEDEDEGEE UniProtKB: Tubulin alpha-4A chain |
-Macromolecule #2: Tubulin beta-2A chain
| Macromolecule | Name: Tubulin beta-2A chain / type: protein_or_peptide / ID: 2 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 51.150961 KDa |
| Recombinant expression | Organism:  Insecta environmental sample (insect) Insecta environmental sample (insect) |
| Sequence | String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS ...String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS VMPSPKVSDT VVEPYNATLS VHQLVENTDE TYSIDNEALY DICFRTLKLT TPTYGDLNHL VSATMSGVTT CL RFPGQLN ADLRKLAVNM VPFPRLHFFM PGFAPLTSRG SQQYRALTVP ELTQQMFDSK NMMAACDPRH GRYLTVAAIF RGR MSMKEV DEQMLNVQNK NSSYFVEWIP NNVKTAVCDI PPRGLKMSAT FIGNSTAIQE LFKRISEQFT AMFRRKAFLH WYTG EGMDE MEFTEAESNM NDLVSEYQQY QDATADEQGE FEEEEGEDEA GGSGGDYKDD DK UniProtKB: Tubulin beta-2A chain |
-Macromolecule #3: Kinesin-1 heavy chain
| Macromolecule | Name: Kinesin-1 heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.721066 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MASMADLAEC NIKVMCRFRP LNESEVNRGD KYIAKFQGED TVVIASKPYA FDRVFQSSTS QEQVYNDCA KKIVKDVLEG YNGTIFAYGQ TSSGKTHTME GKLHDPEGMG IIPRIVQDIF NYIYSMDENL EFHIKVSYFE I YLDKIRDL ...String: MGSSHHHHHH SSGLVPRGSH MASMADLAEC NIKVMCRFRP LNESEVNRGD KYIAKFQGED TVVIASKPYA FDRVFQSSTS QEQVYNDCA KKIVKDVLEG YNGTIFAYGQ TSSGKTHTME GKLHDPEGMG IIPRIVQDIF NYIYSMDENL EFHIKVSYFE I YLDKIRDL LDVSKTNLSV HEDKNRVPYV KGCTERFVCS PDEVMDTIDE GKSNRHVAVT NMNEHSSRSH SIFLINVKQE NT QTEQKLS GKLYLVDLAG SAKVSKTGAE GAVLDEAKNI NKSLSALGNV ISALAEGSTY VPYRDSKMTR ILQDSLGGNC RTT IVICCS PSSYNESETK STLLFGQRAK TIKNTVCVNV ELTAEQWKKK YEKEKE UniProtKB: Kinesin-1 heavy chain |
-Macromolecule #4: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 9 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #5: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER
| Macromolecule | Name: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER / type: ligand / ID: 5 / Number of copies: 9 / Formula: G2P |
|---|---|
| Molecular weight | Theoretical: 521.208 Da |
| Chemical component information |  ChemComp-G2P: |
-Macromolecule #6: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 9 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 6.9 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 36.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 24668 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)