[English] 日本語
 Yorodumi
Yorodumi- EMDB-35104: Structure of beta-arrestin1 in complex with a phosphopeptide corr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
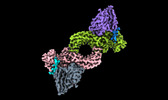
| Title | Structure of beta-arrestin1 in complex with a phosphopeptide corresponding to the human C5a anaphylatoxin chemotactic receptor 1, C5aR1 (Local refine) | |||||||||
 Map data Map data | Full map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / Arrestin / SIGNALING PROTEIN / SIGNALING PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationcomplement component C5a signaling pathway / V2 vasopressin receptor binding / presynapse organization / alpha-1A adrenergic receptor binding / follicle-stimulating hormone receptor binding / TGFBR3 regulates TGF-beta signaling / sensory perception of touch / G alpha (s) signalling events / regulation of inositol trisphosphate biosynthetic process / alpha-1B adrenergic receptor binding ...complement component C5a signaling pathway / V2 vasopressin receptor binding / presynapse organization / alpha-1A adrenergic receptor binding / follicle-stimulating hormone receptor binding / TGFBR3 regulates TGF-beta signaling / sensory perception of touch / G alpha (s) signalling events / regulation of inositol trisphosphate biosynthetic process / alpha-1B adrenergic receptor binding / follicle-stimulating hormone signaling pathway / protein phosphorylated amino acid binding / complement component C5a receptor activity / Lysosome Vesicle Biogenesis / angiotensin receptor binding / response to peptidoglycan / AP-2 adaptor complex binding / Ub-specific processing proteases / MAP2K and MAPK activation / Golgi Associated Vesicle Biogenesis / sensory perception of chemical stimulus / Cargo recognition for clathrin-mediated endocytosis / clathrin adaptor activity / Clathrin-mediated endocytosis / negative regulation of interleukin-8 production / regulation of G protein-coupled receptor signaling pathway / complement receptor mediated signaling pathway / G protein-coupled receptor internalization / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / arrestin family protein binding / mitogen-activated protein kinase kinase binding / positive regulation of neutrophil chemotaxis / Thrombin signalling through proteinase activated receptors (PARs) / response to morphine / clathrin binding / stress fiber assembly / positive regulation of Rho protein signal transduction / positive regulation of macrophage chemotaxis / pseudopodium / amyloid-beta clearance / negative regulation of interleukin-6 production / positive regulation of vascular endothelial growth factor production / positive regulation of receptor internalization / negative regulation of Notch signaling pathway / phototransduction / positive regulation of insulin secretion involved in cellular response to glucose stimulus / cellular defense response / insulin-like growth factor receptor binding / clathrin-coated pit / positive regulation of protein ubiquitination / neutrophil chemotaxis / negative regulation of protein ubiquitination / astrocyte activation / GTPase activator activity / Peptide ligand-binding receptors / secretory granule membrane / Regulation of Complement cascade / nuclear estrogen receptor binding / positive regulation of epithelial cell proliferation / phosphoprotein binding / mRNA transcription by RNA polymerase II / microglial cell activation / G protein-coupled receptor activity / G protein-coupled receptor binding / negative regulation of ERK1 and ERK2 cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cognition / positive regulation of protein phosphorylation / endocytosis / positive regulation of angiogenesis / chemotaxis / apical part of cell / protein transport / positive regulation of cytosolic calcium ion concentration / ubiquitin-dependent protein catabolic process / cytoplasmic vesicle / G alpha (i) signalling events / regulation of apoptotic process / basolateral plasma membrane / phospholipase C-activating G protein-coupled receptor signaling pathway / molecular adaptor activity / dendritic spine / proteasome-mediated ubiquitin-dependent protein catabolic process / negative regulation of neuron apoptotic process / transmembrane transporter binding / postsynaptic membrane / transcription coactivator activity / positive regulation of ERK1 and ERK2 cascade / protein ubiquitination / endosome / positive regulation of MAPK cascade / defense response to Gram-positive bacterium / postsynaptic density / immune response / G protein-coupled receptor signaling pathway / inflammatory response / response to xenobiotic stimulus / signaling receptor binding / positive regulation of cell population proliferation / ubiquitin protein ligase binding Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
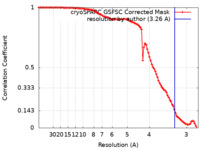
| Method | single particle reconstruction / cryo EM / Resolution: 3.26 Å | |||||||||
 Authors Authors | Maharana J / Sarma P / Yadav MK / Banerjee R / Shukla AK | |||||||||
| Funding support |  India, 1 items India, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Structural snapshots uncover a key phosphorylation motif in GPCRs driving β-arrestin activation. Authors: Jagannath Maharana / Parishmita Sarma / Manish K Yadav / Sayantan Saha / Vinay Singh / Shirsha Saha / Mohamed Chami / Ramanuj Banerjee / Arun K Shukla /   Abstract: Agonist-induced GPCR phosphorylation is a key determinant for the binding and activation of β-arrestins (βarrs). However, it is not entirely clear how different GPCRs harboring divergent ...Agonist-induced GPCR phosphorylation is a key determinant for the binding and activation of β-arrestins (βarrs). However, it is not entirely clear how different GPCRs harboring divergent phosphorylation patterns impart converging active conformation on βarrs leading to broadly conserved functional responses such as desensitization, endocytosis, and signaling. Here, we present multiple cryo-EM structures of activated βarrs in complex with distinct phosphorylation patterns derived from the carboxyl terminus of different GPCRs. These structures help identify a P-X-P-P type phosphorylation motif in GPCRs that interacts with a spatially organized K-K-R-R-K-K sequence in the N-domain of βarrs. Sequence analysis of the human GPCRome reveals the presence of this phosphorylation pattern in a large number of receptors, and its contribution in βarr activation is demonstrated by targeted mutagenesis experiments combined with an intrabody-based conformational sensor. Taken together, our findings provide important structural insights into the ability of distinct GPCRs to activate βarrs through a significantly conserved mechanism. #1:  Journal: Mol.Cell / Year: 2023 Journal: Mol.Cell / Year: 2023Title: Structure of beta-arrestin in complex with a phosphopeptide Authors: Maharana J / Sarma P / Yadav MK / Banerjee R / Shukla AK | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35104.map.gz emd_35104.map.gz | 86 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35104-v30.xml emd-35104-v30.xml emd-35104.xml emd-35104.xml | 22.8 KB 22.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35104_fsc.xml emd_35104_fsc.xml | 9.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_35104.png emd_35104.png | 46 KB | ||
| Filedesc metadata |  emd-35104.cif.gz emd-35104.cif.gz | 6.8 KB | ||
| Others |  emd_35104_half_map_1.map.gz emd_35104_half_map_1.map.gz emd_35104_half_map_2.map.gz emd_35104_half_map_2.map.gz | 84.3 MB 84.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35104 http://ftp.pdbj.org/pub/emdb/structures/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35104 | HTTPS FTP |
-Validation report
| Summary document |  emd_35104_validation.pdf.gz emd_35104_validation.pdf.gz | 956.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35104_full_validation.pdf.gz emd_35104_full_validation.pdf.gz | 955.6 KB | Display | |
| Data in XML |  emd_35104_validation.xml.gz emd_35104_validation.xml.gz | 17.7 KB | Display | |
| Data in CIF |  emd_35104_validation.cif.gz emd_35104_validation.cif.gz | 23 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35104 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35104 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35104 | HTTPS FTP |
-Related structure data
| Related structure data |  8i0nMC  8go8C  8gocC  8gooC  8gp3C  8i0qC  8i0zC  8i10C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35104.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35104.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3667 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map B
| File | emd_35104_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_35104_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine
| Entire | Name: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine |
|---|---|
| Components |
|
-Supramolecule #1: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine
| Supramolecule | Name: Peptide 1 bound beta-arrestin1 in complex with Fab30 - Local refine type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 190 KDa |
-Supramolecule #2: beta-arrestin1
| Supramolecule | Name: beta-arrestin1 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: C5a anaphylatoxin chemotactic receptor 1
| Supramolecule | Name: C5a anaphylatoxin chemotactic receptor 1 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4 / Details: Chemically synthesized |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Synthetically produced: Yes Homo sapiens (human) / Synthetically produced: Yes |
-Supramolecule #4: Fab30
| Supramolecule | Name: Fab30 / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Beta-arrestin-1
| Macromolecule | Name: Beta-arrestin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.088508 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTCA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPCSVTL QPGPEDTGKA CGVDYEVKAF CAENLEEKIH K RNSVRLVI ...String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTCA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPCSVTL QPGPEDTGKA CGVDYEVKAF CAENLEEKIH K RNSVRLVI RKVQYAPERP GPQPTAETTR QFLMSDKPLH LEASLDKEIY YHGEPISVNV HVTNNTNKTV KKIKISVRQY AD ICLFNTA QYKCPVAMEE ADDTVAPSST FCKVYTLTPF LANNREKRGL ALDGKLKHED TNLASSTLLR EGANREILGI IVS YKVKVK LVVSRGGLLG DLASSDVAVE LPFTLMHPKP KEEPPHREVP ESETPVDTNL IELDTNDDDI VFEDFARQRL KGMK DDKDE EDDGTGSPHL NNR UniProtKB: Beta-arrestin-1 |
-Macromolecule #2: Fab30 heavy chain
| Macromolecule | Name: Fab30 heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.512354 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EISEVQLVES GGGLVQPGGS LRLSCAASGF NVYSSSIHWV RQAPGKGLEW VASISSYYGY TYYADSVKGR FTISADTSKN TAYLQMNSL RAEDTAVYYC ARSRQFWYSG LDYWGQGTLV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA ...String: EISEVQLVES GGGLVQPGGS LRLSCAASGF NVYSSSIHWV RQAPGKGLEW VASISSYYGY TYYADSVKGR FTISADTSKN TAYLQMNSL RAEDTAVYYC ARSRQFWYSG LDYWGQGTLV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK THHHHHHHH |
-Macromolecule #3: Fab30 light chain
| Macromolecule | Name: Fab30 light chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.435064 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKR TVAAPSVFIF PPSDSQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD ...String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKR TVAAPSVFIF PPSDSQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD SKDSTYSLSS TLTLSKADYE KHKVYACEVT HQGLSSPVTK SFNRGEC |
-Macromolecule #4: C5a anaphylatoxin chemotactic receptor 1
| Macromolecule | Name: C5a anaphylatoxin chemotactic receptor 1 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.69834 KDa |
| Sequence | String: E(SEP)K(SEP)F(TPO)R(SEP)(TPO)V D(TPO)MAQKTQAV UniProtKB: C5a anaphylatoxin chemotactic receptor 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283.15 K / Instrument: LEICA EM GP / Details: Blotted for 3 seconds before plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 6212 / Average electron dose: 56.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-8i0n: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)