+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Human ATP synthase state 2 (combined) | |||||||||||||||
 マップデータ マップデータ | ||||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | MEMBRANE PROTEIN | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of cell adhesion involved in substrate-bound cell migration / Formation of ATP by chemiosmotic coupling / Cristae formation / estradiol binding / angiostatin binding / ATP biosynthetic process / mitochondrial proton-transporting ATP synthase complex assembly / Mitochondrial protein import / cellular response to interleukin-7 / proton channel activity ...negative regulation of cell adhesion involved in substrate-bound cell migration / Formation of ATP by chemiosmotic coupling / Cristae formation / estradiol binding / angiostatin binding / ATP biosynthetic process / mitochondrial proton-transporting ATP synthase complex assembly / Mitochondrial protein import / cellular response to interleukin-7 / proton channel activity / oxidative phosphorylation / response to copper ion / response to muscle activity / cellular response to cytokine stimulus / negative regulation of endothelial cell proliferation / MHC class I protein binding / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / mitochondrial nucleoid / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / positive regulation of blood vessel endothelial cell migration / response to hyperoxia / cellular response to dexamethasone stimulus / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / substantia nigra development / Mitochondrial protein degradation / cellular response to nitric oxide / proton transmembrane transport / aerobic respiration / cellular response to cAMP / regulation of intracellular pH / generation of precursor metabolites and energy / Transcriptional activation of mitochondrial biogenesis / ADP binding / mitochondrial membrane / lipid metabolic process / fibrillar center / osteoblast differentiation / protease binding / response to ethanol / angiogenesis / nuclear membrane / hydrolase activity / mitochondrial inner membrane / mitochondrial matrix / membrane raft / lipid binding / protein-containing complex binding / structural molecule activity / cell surface / ATP hydrolysis activity / mitochondrion / RNA binding / extracellular exosome / ATP binding / nucleus / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.77 Å | |||||||||||||||
 データ登録者 データ登録者 | Lai Y / Zhang Y / Liu F / Gao Y / Gong H / Rao Z | |||||||||||||||
| 資金援助 |  中国, 4件 中国, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Mol Cell / 年: 2023 ジャーナル: Mol Cell / 年: 2023タイトル: Structure of the human ATP synthase. 著者: Yuezheng Lai / Yuying Zhang / Shan Zhou / Jinxu Xu / Zhanqiang Du / Ziyan Feng / Long Yu / Ziqing Zhao / Weiwei Wang / Yanting Tang / Xiuna Yang / Luke W Guddat / Fengjiang Liu / Yan Gao / ...著者: Yuezheng Lai / Yuying Zhang / Shan Zhou / Jinxu Xu / Zhanqiang Du / Ziyan Feng / Long Yu / Ziqing Zhao / Weiwei Wang / Yanting Tang / Xiuna Yang / Luke W Guddat / Fengjiang Liu / Yan Gao / Zihe Rao / Hongri Gong /   要旨: Biological energy currency ATP is produced by FF-ATP synthase. However, the molecular mechanism for human ATP synthase action remains unknown. Here, we present snapshot images for three main ...Biological energy currency ATP is produced by FF-ATP synthase. However, the molecular mechanism for human ATP synthase action remains unknown. Here, we present snapshot images for three main rotational states and one substate of human ATP synthase using cryoelectron microscopy. These structures reveal that the release of ADP occurs when the β subunit of FF-ATP synthase is in the open conformation, showing how ADP binding is coordinated during synthesis. The accommodation of the symmetry mismatch between F and F motors is resolved by the torsional flexing of the entire complex, especially the γ subunit, and the rotational substep of the c subunit. Water molecules are identified in the inlet and outlet half-channels, suggesting that the proton transfer in these two half-channels proceed via a Grotthus mechanism. Clinically relevant mutations are mapped to the structure, showing that they are mainly located at the subunit-subunit interfaces, thus causing instability of the complex. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_34581.map.gz emd_34581.map.gz | 473.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-34581-v30.xml emd-34581-v30.xml emd-34581.xml emd-34581.xml | 30.7 KB 30.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_34581.png emd_34581.png | 130.1 KB | ||
| Filedesc metadata |  emd-34581.cif.gz emd-34581.cif.gz | 8.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34581 http://ftp.pdbj.org/pub/emdb/structures/EMD-34581 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34581 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34581 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_34581_validation.pdf.gz emd_34581_validation.pdf.gz | 586.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_34581_full_validation.pdf.gz emd_34581_full_validation.pdf.gz | 586.1 KB | 表示 | |
| XML形式データ |  emd_34581_validation.xml.gz emd_34581_validation.xml.gz | 8.3 KB | 表示 | |
| CIF形式データ |  emd_34581_validation.cif.gz emd_34581_validation.cif.gz | 9.7 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34581 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34581 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34581 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34581 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8h9tMC  8h9eC  8h9fC  8h9gC  8h9iC  8h9jC  8h9kC  8h9lC  8h9mC  8h9nC  8h9pC  8h9qC  8h9rC  8h9sC  8h9uC  8h9vC C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_34581.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_34581.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.73 Å | ||||||||||||||||||||||||||||||||||||
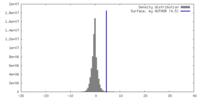
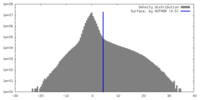
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : Human ATP synthase
+超分子 #1: Human ATP synthase
+分子 #1: ATP synthase F(0) complex subunit B1, mitochondrial
+分子 #2: ATP synthase-coupling factor 6, mitochondrial
+分子 #3: ATP synthase subunit d, mitochondrial
+分子 #4: ATP synthase F(0) complex subunit C1, mitochondrial
+分子 #5: ATP synthase subunit delta, mitochondrial
+分子 #6: ATP synthase subunit epsilon, mitochondrial
+分子 #7: ATP synthase subunit a
+分子 #8: ATP synthase subunit ATP5MJ, mitochondrial
+分子 #9: ATP synthase subunit f, mitochondrial
+分子 #10: ATP synthase subunit g, mitochondrial
+分子 #11: ATP synthase subunit e, mitochondrial
+分子 #12: ATP synthase protein 8
+分子 #13: ATP synthase subunit alpha, mitochondrial
+分子 #14: ATP synthase subunit beta, mitochondrial
+分子 #15: ATP synthase subunit gamma, mitochondrial
+分子 #16: ATP synthase subunit O, mitochondrial
+分子 #17: ADENOSINE-5'-TRIPHOSPHATE
+分子 #18: MAGNESIUM ION
+分子 #19: ADENOSINE-5'-DIPHOSPHATE
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 |
|---|---|
| グリッド | モデル: Quantifoil R1.2/1.3 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | エネルギーフィルター - 名称: TFS Selectris X / エネルギーフィルター - スリット幅: 10 eV |
| 撮影 | フィルム・検出器のモデル: FEI FALCON IV (4k x 4k) 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.4 µm / 最小 デフォーカス(公称値): 1.2 µm |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー
























 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)