+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Lodoxamide-bound GPR35 in complex with G13 | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | GPR35 / G13 / Lodoxamide / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報D5 dopamine receptor binding / regulation of fibroblast migration / Rho-activating G protein-coupled receptor signaling pathway / negative regulation of voltage-gated calcium channel activity / C-X-C chemokine receptor activity / chemokine-mediated signaling pathway / Class A/1 (Rhodopsin-like receptors) / regulation of small GTPase mediated signal transduction / NRAGE signals death through JNK / branching involved in blood vessel morphogenesis ...D5 dopamine receptor binding / regulation of fibroblast migration / Rho-activating G protein-coupled receptor signaling pathway / negative regulation of voltage-gated calcium channel activity / C-X-C chemokine receptor activity / chemokine-mediated signaling pathway / Class A/1 (Rhodopsin-like receptors) / regulation of small GTPase mediated signal transduction / NRAGE signals death through JNK / branching involved in blood vessel morphogenesis / CDC42 GTPase cycle / regulation of postsynapse assembly / Rho protein signal transduction / cytoskeleton organization / RAC1 GTPase cycle / guanyl-nucleotide exchange factor activity / brush border membrane / G protein-coupled receptor activity / platelet activation / regulation of blood pressure / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / adenylate cyclase-activating dopamine receptor signaling pathway / melanosome / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / regulation of cell shape / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / G protein activity / positive regulation of cytosolic calcium ion concentration / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / in utero embryonic development / Ras protein signal transduction / cell differentiation / Extra-nuclear estrogen signaling / cell population proliferation / postsynapse / G protein-coupled receptor signaling pathway / lysosomal membrane / focal adhesion / GTPase activity / synapse / GTP binding / protein-containing complex binding / signal transduction / extracellular exosome / metal ion binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.2 Å | |||||||||
 データ登録者 データ登録者 | Yuan Q / Duan J / Liu Q / Xu HE / Jiang Y | |||||||||
| 資金援助 |  中国, 1件 中国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Cell Discov / 年: 2022 ジャーナル: Cell Discov / 年: 2022タイトル: Insights into divalent cation regulation and G-coupling of orphan receptor GPR35. 著者: Jia Duan / Qiufeng Liu / Qingning Yuan / Yujie Ji / Shengnan Zhu / Yangxia Tan / Xinheng He / Youwei Xu / Jingjing Shi / Xi Cheng / Hualiang Jiang / H Eric Xu / Yi Jiang /  要旨: Endogenous ions play important roles in the function and pharmacology of G protein-coupled receptors (GPCRs) with limited atomic evidence. In addition, compared with G protein subtypes G, G, and G, ...Endogenous ions play important roles in the function and pharmacology of G protein-coupled receptors (GPCRs) with limited atomic evidence. In addition, compared with G protein subtypes G, G, and G, insufficient structural evidence is accessible to understand the coupling mechanism of G protein by GPCRs. Orphan receptor GPR35, which is predominantly expressed in the gastrointestinal tract and is closely related to inflammatory bowel diseases (IBDs), stands out as a prototypical receptor for investigating ionic modulation and G coupling. Here we report a cryo-electron microscopy structure of G-coupled GPR35 bound to an anti-allergic drug, lodoxamide. This structure reveals a novel divalent cation coordination site and a unique ionic regulatory mode of GPR35 and also presents a highly positively charged binding pocket and the complementary electrostatic ligand recognition mode, which explain the promiscuity of acidic ligand binding by GPR35. Structural comparison of the GPR35-G complex with other G protein subtypes-coupled GPCRs reveals a notable movement of the C-terminus of α5 helix of the Gα subunit towards the receptor core and the least outward displacement of the cytoplasmic end of GPR35 TM6. A featured 'methionine pocket' contributes to the G coupling by GPR35. Together, our findings provide a structural basis for divalent cation modulation, ligand recognition, and subsequent G protein coupling of GPR35 and offer a new opportunity for designing GPR35-targeted drugs for the treatment of IBDs. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_34549.map.gz emd_34549.map.gz | 56.1 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-34549-v30.xml emd-34549-v30.xml emd-34549.xml emd-34549.xml | 19.7 KB 19.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_34549.png emd_34549.png | 38.6 KB | ||
| Filedesc metadata |  emd-34549.cif.gz emd-34549.cif.gz | 6.6 KB | ||
| その他 |  emd_34549_half_map_1.map.gz emd_34549_half_map_1.map.gz emd_34549_half_map_2.map.gz emd_34549_half_map_2.map.gz | 49.8 MB 49.7 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34549 http://ftp.pdbj.org/pub/emdb/structures/EMD-34549 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34549 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34549 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_34549_validation.pdf.gz emd_34549_validation.pdf.gz | 872 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_34549_full_validation.pdf.gz emd_34549_full_validation.pdf.gz | 871.5 KB | 表示 | |
| XML形式データ |  emd_34549_validation.xml.gz emd_34549_validation.xml.gz | 12.1 KB | 表示 | |
| CIF形式データ |  emd_34549_validation.cif.gz emd_34549_validation.cif.gz | 14.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34549 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34549 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34549 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34549 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8h8jMC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_34549.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_34549.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
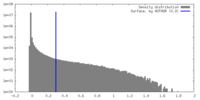
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_34549_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
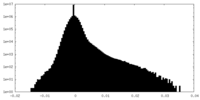
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_34549_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
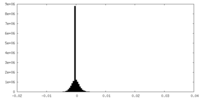
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Lodoxamide-GPR35-G13 complex
| 全体 | 名称: Lodoxamide-GPR35-G13 complex |
|---|---|
| 要素 |
|
-超分子 #1: Lodoxamide-GPR35-G13 complex
| 超分子 | 名称: Lodoxamide-GPR35-G13 complex / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#5 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Guanine nucleotide-binding protein subunit alpha-13
| 分子 | 名称: Guanine nucleotide-binding protein subunit alpha-13 / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 42.423633 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGCTLSAEDK AAVERSKMID KCLSREKTYV KRLVKILLLG AGESGKSTFL KQMRIIHGQD FDQRAREEFR PTIYSNVIKG MRVLVDARE KLHIPWGDNS NQQHGDKMMS FDTRAPMAAQ GMVETRVFLQ YLPAIRALWA DSGIQNAYDR RREFQLGESV K YFLDNLDK ...文字列: MGCTLSAEDK AAVERSKMID KCLSREKTYV KRLVKILLLG AGESGKSTFL KQMRIIHGQD FDQRAREEFR PTIYSNVIKG MRVLVDARE KLHIPWGDNS NQQHGDKMMS FDTRAPMAAQ GMVETRVFLQ YLPAIRALWA DSGIQNAYDR RREFQLGESV K YFLDNLDK LGEPDYIPSQ QDILLARRPT KGIHEYDFEI KNVPFKMVDV GGQRSERKRW FECFDSVTSI LFLVSSSEFD QV LMEDRLT NRLTESLNIF ETIVNNRVFS NVSIILFLNK TDLLEEKVQI VSIKDYFLEF EGDPHCLRDV QKFLVECFRN KRR DQQQKP LYHHFTTAIN TENIRLVFRD VKDTILHDNL KQLMLQ UniProtKB: Guanine nucleotide-binding protein subunit alpha-13 |
-分子 #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 40.226992 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD ...文字列: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD TTCALWDIET GQQTTTFTGH TGDVMSLSLA PDTRLFVSGA CDASAKLWDV REGMCRQTFT GHESDINAIC FF PNGNAFA TGSDDATCRL FDLRADQELM TYSHDNIICG ITSVSFSKSG RLLLAGYDDF NCNVWDALKA DRAGVLAGHD NRV SCLGVT DDGMAVATGS WDSFLKIWNG SSGGGGSGGG GSSGVSGWRL FKKIS UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #3: G-protein coupled receptor 35
| 分子 | 名称: G-protein coupled receptor 35 / タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 33.972047 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: NGTYNTCGSS DLTWPPAIKL GFYAYLGVLL VLGLLLNSLA LWVFCCRMQQ WTETRIYMTN LAVADLCLLC TLPFVLHSLR DTSDTPLCQ LSQGIYLTNR YMSISLVTAI AVDRYVAVRH PLRARGLRSP RQAAAVCAVL WVLVIGSLVA RWLLGIQEGG F CFRSTRHN ...文字列: NGTYNTCGSS DLTWPPAIKL GFYAYLGVLL VLGLLLNSLA LWVFCCRMQQ WTETRIYMTN LAVADLCLLC TLPFVLHSLR DTSDTPLCQ LSQGIYLTNR YMSISLVTAI AVDRYVAVRH PLRARGLRSP RQAAAVCAVL WVLVIGSLVA RWLLGIQEGG F CFRSTRHN FNSMAFPLLG FYLPLAVVVF CSLKVVTALA QRPPTDVGQA EATRKAARMV WANLLVFVVC FLPLHVGLTV RL AVGWNAC ALLETIRRAL YITSKLSDAN CCLDAICYYY MAKEFQEASA LAVAPSAKAH KSQDSLCVTL A UniProtKB: G-protein coupled receptor 35 |
-分子 #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 7.861143 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #5: scFv16
| 分子 | 名称: scFv16 / タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 26.277299 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSADIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...文字列: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSADIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL |
-分子 #6: 2,2'-[(2-chloro-5-cyano-1,3-phenylene)bis(azanediyl)]bis(oxoaceti...
| 分子 | 名称: 2,2'-[(2-chloro-5-cyano-1,3-phenylene)bis(azanediyl)]bis(oxoacetic acid) タイプ: ligand / ID: 6 / コピー数: 1 / 式: WYB |
|---|---|
| 分子量 | 理論値: 311.635 Da |
| Chemical component information |  ChemComp-WYB: |
-分子 #7: CALCIUM ION
| 分子 | 名称: CALCIUM ION / タイプ: ligand / ID: 7 / コピー数: 1 / 式: CA |
|---|---|
| 分子量 | 理論値: 40.078 Da |
-分子 #8: CHOLESTEROL
| 分子 | 名称: CHOLESTEROL / タイプ: ligand / ID: 8 / コピー数: 3 / 式: CLR |
|---|---|
| 分子量 | 理論値: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.04 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 0.8 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: OTHER |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.2 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 591246 |
| 初期 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
 ムービー
ムービー コントローラー
コントローラー





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)