+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of BAP1-ASXL1 bound to nucleosome | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||||||||
 Authors Authors | Ge W / Yu C / Xu RM | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Basis of the H2AK119 specificity of the Polycomb repressive deubiquitinase. Authors: Weiran Ge / Cong Yu / Jingjing Li / Zhenyu Yu / Xiaorong Li / Yan Zhang / Chao-Pei Liu / Yingfeng Li / Changlin Tian / Xinzheng Zhang / Guohong Li / Bing Zhu / Rui-Ming Xu /  Abstract: Repression of gene expression by protein complexes of the Polycomb group is a fundamental mechanism that governs embryonic development and cell-type specification. The Polycomb repressive ...Repression of gene expression by protein complexes of the Polycomb group is a fundamental mechanism that governs embryonic development and cell-type specification. The Polycomb repressive deubiquitinase (PR-DUB) complex removes the ubiquitin moiety from monoubiquitinated histone H2A K119 (H2AK119ub1) on the nucleosome, counteracting the ubiquitin E3 ligase activity of Polycomb repressive complex 1 (PRC1) to facilitate the correct silencing of genes by Polycomb proteins and safeguard active genes from inadvertent silencing by PRC1 (refs. ). The intricate biological function of PR-DUB requires accurate targeting of H2AK119ub1, but PR-DUB can deubiquitinate monoubiquitinated free histones and peptide substrates indiscriminately; the basis for its exquisite nucleosome-dependent substrate specificity therefore remains unclear. Here we report the cryo-electron microscopy structure of human PR-DUB, composed of BAP1 and ASXL1, in complex with the chromatosome. We find that ASXL1 directs the binding of the positively charged C-terminal extension of BAP1 to nucleosomal DNA and histones H3-H4 near the dyad, an addition to its role in forming the ubiquitin-binding cleft. Furthermore, a conserved loop segment of the catalytic domain of BAP1 is situated near the H2A-H2B acidic patch. This distinct nucleosome-binding mode displaces the C-terminal tail of H2A from the nucleosome surface, and endows PR-DUB with the specificity for H2AK119ub1. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34432.map.gz emd_34432.map.gz | 5.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34432-v30.xml emd-34432-v30.xml emd-34432.xml emd-34432.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34432.png emd_34432.png | 54.4 KB | ||
| Masks |  emd_34432_msk_1.map emd_34432_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_34432_additional_1.map.gz emd_34432_additional_1.map.gz emd_34432_additional_2.map.gz emd_34432_additional_2.map.gz emd_34432_half_map_1.map.gz emd_34432_half_map_1.map.gz emd_34432_half_map_2.map.gz emd_34432_half_map_2.map.gz | 49.5 MB 1002.5 KB 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34432 http://ftp.pdbj.org/pub/emdb/structures/EMD-34432 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34432 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34432 | HTTPS FTP |
-Validation report
| Summary document |  emd_34432_validation.pdf.gz emd_34432_validation.pdf.gz | 943.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34432_full_validation.pdf.gz emd_34432_full_validation.pdf.gz | 943.1 KB | Display | |
| Data in XML |  emd_34432_validation.xml.gz emd_34432_validation.xml.gz | 11 KB | Display | |
| Data in CIF |  emd_34432_validation.cif.gz emd_34432_validation.cif.gz | 12.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34432 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34432 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34432 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34432 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34432.map.gz / Format: CCP4 / Size: 6.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34432.map.gz / Format: CCP4 / Size: 6.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_34432_msk_1.map emd_34432_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
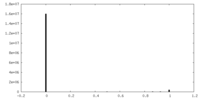
| Density Histograms |
-Additional map: #2
| File | emd_34432_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
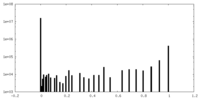
| Density Histograms |
-Additional map: #1
| File | emd_34432_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
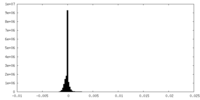
| Density Histograms |
-Half map: #1
| File | emd_34432_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
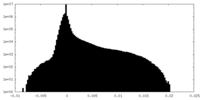
| Density Histograms |
-Half map: #2
| File | emd_34432_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of BAP1-ASXL1 bound to nucleosome
| Entire | Name: Cryo-EM structure of BAP1-ASXL1 bound to nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of BAP1-ASXL1 bound to nucleosome
| Supramolecule | Name: Cryo-EM structure of BAP1-ASXL1 bound to nucleosome / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#10 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 284 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software: (Name: cryoSPARC (ver. 3.2), RELION (ver. 3.0.8)) / Number images used: 56370 |
|---|---|
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|
 Movie
Movie Controller
Controller










 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)