[English] 日本語
 Yorodumi
Yorodumi- EMDB-34083: GluK1-1a extracellular domain captured in SYM2081 bound desensiti... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GluK1-1a extracellular domain captured in SYM2081 bound desensitized state | |||||||||
 Map data Map data | GluK1-1a extracellular domain captured in desensitized state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Kainate receptor / GluK1-1a splice variant / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of synaptic transmission, glutamatergic / glutamate-gated receptor activity / regulation of membrane potential / terminal bouton / postsynaptic membrane / dendrite Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 8.01 Å | |||||||||
 Authors Authors | Dhingra S / Kumar J | |||||||||
| Funding support |  India, 1 items India, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: Functional Implications of the Exon 9 Splice Insert in GluK1 Kainate Receptors Authors: Dhingra S / Chopade PM / Vinnakota R / Kumar J | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34083.map.gz emd_34083.map.gz | 32.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34083-v30.xml emd-34083-v30.xml emd-34083.xml emd-34083.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34083.png emd_34083.png | 51.3 KB | ||
| Masks |  emd_34083_msk_1.map emd_34083_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-34083.cif.gz emd-34083.cif.gz | 6.8 KB | ||
| Others |  emd_34083_additional_1.map.gz emd_34083_additional_1.map.gz emd_34083_half_map_1.map.gz emd_34083_half_map_1.map.gz emd_34083_half_map_2.map.gz emd_34083_half_map_2.map.gz | 57.6 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34083 http://ftp.pdbj.org/pub/emdb/structures/EMD-34083 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34083 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34083 | HTTPS FTP |
-Related structure data
| Related structure data |  7ysvMC  7ysjC  8gprC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34083.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34083.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GluK1-1a extracellular domain captured in desensitized state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.38 Å | ||||||||||||||||||||||||||||||||||||
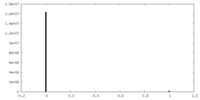
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_34083_msk_1.map emd_34083_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
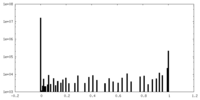
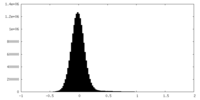
| Density Histograms |
-Additional map: GluK1-1a extracellular domain captured in desensitized state additional EM...
| File | emd_34083_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GluK1-1a extracellular domain captured in desensitized state_additional EM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
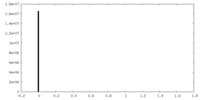
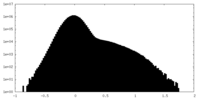
| Density Histograms |
-Half map: GluK1-1a extracellular domain captured in desensitized state halfmap A...
| File | emd_34083_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GluK1-1a extracellular domain captured in desensitized state halfmap_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
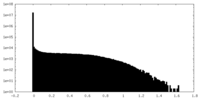
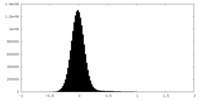
| Density Histograms |
-Half map: GluK1-1a extracellular domain captured in desensitized state halfmap B...
| File | emd_34083_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GluK1-1a extracellular domain captured in desensitized state halfmap_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
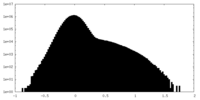
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of GluK1-1a extracellular domain with SYM2081
| Entire | Name: Complex of GluK1-1a extracellular domain with SYM2081 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of GluK1-1a extracellular domain with SYM2081
| Supramolecule | Name: Complex of GluK1-1a extracellular domain with SYM2081 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 390 KDa |
-Macromolecule #1: Glutamate receptor
| Macromolecule | Name: Glutamate receptor / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 95.606672 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVLRIGGIFE TVENEPVNVE ELAFKFAVTS INRNRTLMPN TTLTYDIQRI NLFDSFEASR RACDQLALGV AALFGPSHSS SVSAVQSIC NALEVPHIQT RWKHPSVDSR DLFYINLYPD YAAISRAVLD LVLYYNWKTV TVVYEDSTGL IRLQELIKAP S RYNIKIKI ...String: QVLRIGGIFE TVENEPVNVE ELAFKFAVTS INRNRTLMPN TTLTYDIQRI NLFDSFEASR RACDQLALGV AALFGPSHSS SVSAVQSIC NALEVPHIQT RWKHPSVDSR DLFYINLYPD YAAISRAVLD LVLYYNWKTV TVVYEDSTGL IRLQELIKAP S RYNIKIKI RQLPPANKDA KPLLKEMKKS KEFYVIFDCS HETAAEILKQ ILFMGMMTEY YHYFFTTLDL FALDLELYRY SG VNMTGFR LLNIDNPHVS SIIEKWSMER LQAPPRPETG LLDGMMTTEA ALMYDAVYMV AIASHRASQL TVSSLQCHRH KPW RLGPRF MNLIKEARWD GLTGRITFNK TDGLRKDFDL DIISLKEEGT EKASGEVSKH LYKVWKKIGI WNSNSGLNMT DGNR DRSNN ITDSLANRTL IVTTILEEPY VMYRKSDKPL YGNDRFEGYC LDLLKELSNI LGFLYDVKLV PDGKYGAQND KGEWN GMVK ELIDHRADLA VAPLTITYVR EKVIDFSKPF MTLGISILYR KPNGTNPGVF SFLNPLSPDI WMYVLLAYLG VSVVLF VIA RFTPYEWYNP HPCNPDSDVV ENNFTLLNSF WFGVGALMQQ GSELMPKALS TRIVGGIWWF FTLIIISSYT ANLAAFL TV ERMESPIDSA DDLAKQTKIE YGAVRDGSTM TFFKKSKIST YEKMWAFMSS RQQSALVKNS DEGIQRVLTT DYALLMES T SIEYVTQRNC NLTQIGGLID SKGYGVGTPI GSPYRDKITI AILQLQEEGK LHMMKEKWWR GNGCPEEDSK EASALGVEN IGGIFIVLAA GLVLSVFVAI GEFLYKSRKN NDVEQHYLVP R UniProtKB: Glutamate receptor |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.57 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 1100 / Average exposure time: 60.0 sec. / Average electron dose: 19.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)