[English] 日本語
 Yorodumi
Yorodumi- EMDB-33963: Structure of human SGLT2-MAP17 complex bound with substrate AMG i... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human SGLT2-MAP17 complex bound with substrate AMG in the occluded conformation | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | glucose transporter / SGLT / sodium glucose transporter / membrane protein / PROTEIN TRANSPORT | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationlow-affinity D-glucose:sodium symporter activity / Defective SLC5A2 causes renal glucosuria (GLYS1) / alpha-glucoside transport / alpha-glucoside transmembrane transporter activity / D-glucose:sodium symporter activity / renal D-glucose absorption / hexose transmembrane transport / D-glucose import across plasma membrane / Cellular hexose transport / D-glucose transmembrane transporter activity ...low-affinity D-glucose:sodium symporter activity / Defective SLC5A2 causes renal glucosuria (GLYS1) / alpha-glucoside transport / alpha-glucoside transmembrane transporter activity / D-glucose:sodium symporter activity / renal D-glucose absorption / hexose transmembrane transport / D-glucose import across plasma membrane / Cellular hexose transport / D-glucose transmembrane transporter activity / sodium ion import across plasma membrane / sodium ion transport / carbohydrate metabolic process / apical plasma membrane / extracellular exosome / metal ion binding / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
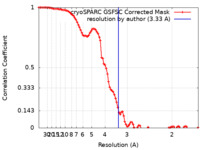
| Method | single particle reconstruction / cryo EM / Resolution: 3.33 Å | ||||||||||||
 Authors Authors | Chen L / Niu Y / Cui W | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structures of human SGLT in the occluded state reveal conformational changes during sugar transport. Authors: Wenhao Cui / Yange Niu / Zejian Sun / Rui Liu / Lei Chen /  Abstract: Sodium-Glucose Cotransporters (SGLT) mediate the uphill uptake of extracellular sugars and play fundamental roles in sugar metabolism. Although their structures in inward-open and outward-open ...Sodium-Glucose Cotransporters (SGLT) mediate the uphill uptake of extracellular sugars and play fundamental roles in sugar metabolism. Although their structures in inward-open and outward-open conformations are emerging from structural studies, the trajectory of how SGLTs transit from the outward-facing to the inward-facing conformation remains unknown. Here, we present the cryo-EM structures of human SGLT1 and SGLT2 in the substrate-bound state. Both structures show an occluded conformation, with not only the extracellular gate but also the intracellular gate tightly sealed. The sugar substrate are caged inside a cavity surrounded by TM1, TM2, TM3, TM6, TM7, and TM10. Further structural analysis reveals the conformational changes associated with the binding and release of substrates. These structures fill a gap in our understanding of the structural mechanisms of SGLT transporters. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33963.map.gz emd_33963.map.gz | 7.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33963-v30.xml emd-33963-v30.xml emd-33963.xml emd-33963.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33963_fsc.xml emd_33963_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_33963.png emd_33963.png | 40.8 KB | ||
| Masks |  emd_33963_msk_1.map emd_33963_msk_1.map | 83.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-33963.cif.gz emd-33963.cif.gz | 6.8 KB | ||
| Others |  emd_33963_additional_1.map.gz emd_33963_additional_1.map.gz emd_33963_additional_2.map.gz emd_33963_additional_2.map.gz emd_33963_half_map_1.map.gz emd_33963_half_map_1.map.gz emd_33963_half_map_2.map.gz emd_33963_half_map_2.map.gz | 78.9 MB 41.3 MB 77.7 MB 77.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33963 http://ftp.pdbj.org/pub/emdb/structures/EMD-33963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33963 | HTTPS FTP |
-Validation report
| Summary document |  emd_33963_validation.pdf.gz emd_33963_validation.pdf.gz | 842.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33963_full_validation.pdf.gz emd_33963_full_validation.pdf.gz | 841.9 KB | Display | |
| Data in XML |  emd_33963_validation.xml.gz emd_33963_validation.xml.gz | 17.5 KB | Display | |
| Data in CIF |  emd_33963_validation.cif.gz emd_33963_validation.cif.gz | 22.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33963 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33963 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33963 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33963 | HTTPS FTP |
-Related structure data
| Related structure data |  7ynjMC  7yniC  7ynkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33963.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33963.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.821 Å | ||||||||||||||||||||||||||||||||||||
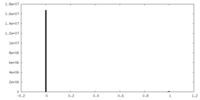
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_33963_msk_1.map emd_33963_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
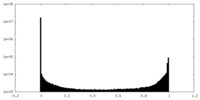
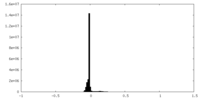
| Density Histograms |
-Additional map: #2
| File | emd_33963_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
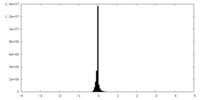
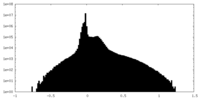
| Density Histograms |
-Additional map: #1
| File | emd_33963_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
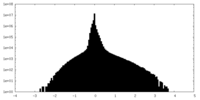
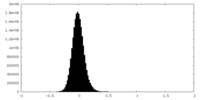
| Density Histograms |
-Half map: #2
| File | emd_33963_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
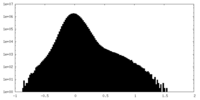
| Density Histograms |
-Half map: #1
| File | emd_33963_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human SGLT2-MAP17 complex
| Entire | Name: human SGLT2-MAP17 complex |
|---|---|
| Components |
|
-Supramolecule #1: human SGLT2-MAP17 complex
| Supramolecule | Name: human SGLT2-MAP17 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sodium/glucose cotransporter 2
| Macromolecule | Name: Sodium/glucose cotransporter 2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 72.949414 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEEHTEAGSA PEMGAQKALI DNPADILVIA AYFLLVIGVG LWSMCRTNRG TVGGYFLAGR SMVWWPVGAS LFASNIGSGH FVGLAGTGA ASGLAVAGFE WNALFVVLLL GWLFAPVYLT AGVITMPQYL RKRFGGRRIR LYLSVLSLFL YIFTKISVDM F SGAVFIQQ ...String: MEEHTEAGSA PEMGAQKALI DNPADILVIA AYFLLVIGVG LWSMCRTNRG TVGGYFLAGR SMVWWPVGAS LFASNIGSGH FVGLAGTGA ASGLAVAGFE WNALFVVLLL GWLFAPVYLT AGVITMPQYL RKRFGGRRIR LYLSVLSLFL YIFTKISVDM F SGAVFIQQ ALGWNIYASV IALLGITMIY TVTGGLAALM YTDTVQTFVI LGGACILMGY AFHEVGGYSG LFDKYLGAAT SL TVSEDPA VGNISSFCYR PRPDSYHLLR HPVTGDLPWP ALLLGLTIVS GWYWCSDQVI VQRCLAGKSL THIKAGCILC GYL KLTPMF LMVMPGMISR ILYPDEVACV VPEVCRRVCG TEVGCSNIAY PRLVVKLMPN GLRGLMLAVM LAALMSSLAS IFNS SSTLF TMDIYTRLRP RAGDRELLLV GRLWVVFIVV VSVAWLPVVQ AAQGGQLFDY IQAVSSYLAP PVSAVFVLAL FVPRV NEQG AFWGLIGGLL MGLARLIPEF SFGSGSCVQP SACPAFLCGV HYLYFAIVLF FCSGLLTLTV SLCTAPIPRK HLHRLV FSL RHSKEEREDL DADEQQGSSL PVQNGCPESA MEMNEPQAPA PSLFRQCLLW FCGMSRGGVG SPPPLTQEEA AAAARRL ED ISEDPSWARV VNLNALLMMA VAVFLWGFYA UniProtKB: Sodium/glucose cotransporter 2 |
-Macromolecule #2: PDZK1-interacting protein 1
| Macromolecule | Name: PDZK1-interacting protein 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.235 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSALSLLILG LLTAVPPASC QQGLGNLQPW MQGLIAVAVF LVLVAIAFAV NHFWCQEEPE PAHMILTVGN KADGVLVGTD GRYSSMAAS FRSSEHENAY ENVPEEEGKV RSTPM UniProtKB: PDZK1-interacting protein 1 |
-Macromolecule #3: methyl alpha-D-glucopyranoside
| Macromolecule | Name: methyl alpha-D-glucopyranoside / type: ligand / ID: 3 / Number of copies: 1 / Formula: GYP |
|---|---|
| Molecular weight | Theoretical: 194.182 Da |
| Chemical component information |  ChemComp-GYP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)