[English] 日本語
 Yorodumi
Yorodumi- EMDB-33810: Structure of the Spring Viraemia of Carp Virus ribonucleoprotein ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Spring Viraemia of Carp Virus ribonucleoprotein Complex | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Ribonucleoprotein / Complex / VIRUS / VIRUS LIKE PARTICLE-RNA complex | ||||||||||||
| Biological species |  Sprivirus cyprinus / Sprivirus cyprinus /  Trichoplusia ni (cabbage looper) / Trichoplusia ni (cabbage looper) /  Sprivivirus cyprinus Sprivivirus cyprinus | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||||||||
 Authors Authors | Liu B / Wang ZX / Yang T / Yu DQ / Ouyang Q | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Virol / Year: 2023 Journal: J Virol / Year: 2023Title: Structure of the Spring Viraemia of Carp Virus Ribonucleoprotein Complex Reveals Its Assembly Mechanism and Application in Antiviral Drug Screening. Authors: Zhao-Xi Wang / Bing Liu / Tian Yang / Daqi Yu / Chu Zhang / Liming Zheng / Jin Xie / Bin Liu / Mengxi Liu / Hailin Peng / Luhua Lai / Qi Ouyang / Songying Ouyang / Yong-An Zhang /  Abstract: Spring viremia of carp virus (SVCV) is a highly pathogenic infecting the common carp, yet neither a vaccine nor effective therapies are available to treat spring viremia of carp (SVC). Like all ...Spring viremia of carp virus (SVCV) is a highly pathogenic infecting the common carp, yet neither a vaccine nor effective therapies are available to treat spring viremia of carp (SVC). Like all negative-sense viruses, SVCV contains an RNA genome that is encapsidated by the nucleoprotein (N) in the form of a ribonucleoprotein (RNP) complex, which serves as the template for viral replication and transcription. Here, the three-dimensional (3D) structure of SVCV RNP was resolved through cryo-electron microscopy (cryo-EM) at a resolution of 3.7 Å. RNP assembly was stabilized by N and C loops; RNA was wrapped in the groove between the N and C lobes with 9 nt nucleotide per protomer. Combined with mutational analysis, our results elucidated the mechanism of RNP formation. The RNA binding groove of SVCV N was used as a target for drug virtual screening, and it was found suramin had a good antiviral effect. This study provided insights into RNP assembly, and anti-SVCV drug screening was performed on the basis of this structure, providing a theoretical basis and efficient drug screening method for the prevention and treatment of SVC. Aquaculture accounts for about 70% of global aquatic products, and viral diseases severely harm the development of aquaculture industry. Spring viremia of carp virus (SVCV) is the pathogen causing highly contagious spring viremia of carp (SVC) disease in cyprinids, especially common carp (), yet neither a vaccine nor effective therapies are available to treat this disease. In this study, we have elucidated the mechanism of SVCV ribonucleoprotein complex (RNP) formation by resolving the 3D structure of SVCV RNP and screened antiviral drugs based on the structure. It is found that suramin could competitively bind to the RNA binding groove and has good antiviral effects both and . Our study provides a template for rational drug discovery efforts to treat and prevent SVCV infections. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33810.map.gz emd_33810.map.gz | 3.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33810-v30.xml emd-33810-v30.xml emd-33810.xml emd-33810.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33810.png emd_33810.png | 89.7 KB | ||
| Masks |  emd_33810_msk_1.map emd_33810_msk_1.map | 18.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-33810.cif.gz emd-33810.cif.gz | 6.8 KB | ||
| Others |  emd_33810_half_map_1.map.gz emd_33810_half_map_1.map.gz emd_33810_half_map_2.map.gz emd_33810_half_map_2.map.gz | 13.7 MB 13.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33810 http://ftp.pdbj.org/pub/emdb/structures/EMD-33810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33810 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33810.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33810.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||||||||||||||||||
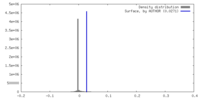
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_33810_msk_1.map emd_33810_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
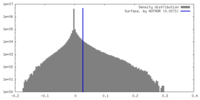
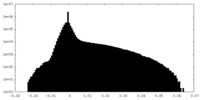
| Density Histograms |
-Half map: #2
| File | emd_33810_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
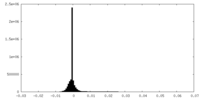
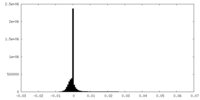
| Density Histograms |
-Half map: #1
| File | emd_33810_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
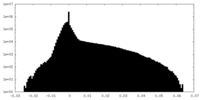
| Density Histograms |
- Sample components
Sample components
-Entire : ribonucleoprotein complex of the spring viraemia carp virus
| Entire | Name: ribonucleoprotein complex of the spring viraemia carp virus |
|---|---|
| Components |
|
-Supramolecule #1: ribonucleoprotein complex of the spring viraemia carp virus
| Supramolecule | Name: ribonucleoprotein complex of the spring viraemia carp virus type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Sprivirus cyprinus Sprivirus cyprinus |
-Supramolecule #2: nucleoprotein
| Supramolecule | Name: nucleoprotein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
-Supramolecule #3: RNA
| Supramolecule | Name: RNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Nucleoprotein
| Macromolecule | Name: Nucleoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 11 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Sprivivirus cyprinus Sprivivirus cyprinus |
| Molecular weight | Theoretical: 46.62116 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: RIKTNAAVAA VLPANEDQAD YPSTFFEGGN EIRLYVNRGE KLDVLRQYVY MGLVEKNCRI QHVNAYLYAV LKGERELLEA DWDSFGHKI GIQGDKIGPF NLVRVEDIPD GLPDGKLNAE VSAEDDAWLP LFLLGLYRVG RASETAYRTL LMESLIKQCK A IKSDWVSP ...String: RIKTNAAVAA VLPANEDQAD YPSTFFEGGN EIRLYVNRGE KLDVLRQYVY MGLVEKNCRI QHVNAYLYAV LKGERELLEA DWDSFGHKI GIQGDKIGPF NLVRVEDIPD GLPDGKLNAE VSAEDDAWLP LFLLGLYRVG RASETAYRTL LMESLIKQCK A IKSDWVSP VTATHKYFDV WGNDGNYLKI VACVDMFYNH FKKSIKATFR WGTIVSRFKD CAALATLGHV VKITGLTIEE VF TWVLQTE VADELVKMMK PGQEIDKSTS YMPYLIDMGI SAKSPYSTIK NPSFHFWGQL VAALCRSKRA LNARQPDEID SMS ISNASL LMAYALGSSP DIEQQFSTGN TYRKPPKEAS YLVSEEPKNR SVVEWIAWYS DVDNKPTDDM LMMAKRVAGT ISGP RDNSV GKWIKQTYG |
-Macromolecule #2: RNA (99-mer)
| Macromolecule | Name: RNA (99-mer) / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Molecular weight | Theoretical: 30.26548 KDa |
| Sequence | String: UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUUU UUUUUUUUU UUUUUUUUUU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: GRAPHENE / Support film - topology: CONTINUOUS |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 300 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7yg7: |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)