+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the monomeric human CAF1LC-H3-H4 complex | |||||||||||||||||||||||||||||||||
 Map data Map data | map | |||||||||||||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||||||||||||||
 Keywords Keywords | Histone chaperone / Chromatin assembly factor / REPLICATION | |||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationCAF-1 complex / chromo shadow domain binding / NuRD complex / NURF complex / regulation of cell fate specification / negative regulation of stem cell population maintenance / DNA replication-dependent chromatin assembly / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / ESC/E(Z) complex / regulation of stem cell differentiation ...CAF-1 complex / chromo shadow domain binding / NuRD complex / NURF complex / regulation of cell fate specification / negative regulation of stem cell population maintenance / DNA replication-dependent chromatin assembly / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / ESC/E(Z) complex / regulation of stem cell differentiation / Polo-like kinase mediated events / Transcription of E2F targets under negative control by DREAM complex / ATPase complex / G1/S-Specific Transcription / Sin3-type complex / Transcriptional Regulation by E2F6 / histone deacetylase complex / positive regulation of stem cell population maintenance / RNA Polymerase I Transcription Initiation / G0 and Early G1 / Cyclin E associated events during G1/S transition / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / negative regulation of megakaryocyte differentiation / Cyclin A:Cdk2-associated events at S phase entry / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / Regulation of TP53 Activity through Acetylation / CENP-A containing nucleosome / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / negative regulation of cell migration / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Regulation of PTEN gene transcription / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / negative regulation of transforming growth factor beta receptor signaling pathway / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / G2/M DNA damage checkpoint / brain development / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / histone deacetylase binding / Activation of anterior HOX genes in hindbrain development during early embryogenesis / RMTs methylate histone arginines / Transcriptional regulation of granulopoiesis / HCMV Early Events / structural constituent of chromatin / unfolded protein binding / nucleosome / nucleosome assembly / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / histone binding / Oxidative Stress Induced Senescence / gene expression / Estrogen-dependent gene expression / Potential therapeutics for SARS / DNA replication / chromosome, telomeric region / cadherin binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / chromatin remodeling Similarity search - Function | |||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||||||||||||||||||||||||||
 Authors Authors | Liu CP / Yu ZY / Yu C / Xu RM | |||||||||||||||||||||||||||||||||
| Funding support |  China, 10 items China, 10 items
| |||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Structural insights into histone binding and nucleosome assembly by chromatin assembly factor-1. Authors: Chao-Pei Liu / Zhenyu Yu / Jun Xiong / Jie Hu / Aoqun Song / Dongbo Ding / Cong Yu / Na Yang / Mingzhu Wang / Juan Yu / Peini Hou / Kangning Zeng / Zhenyu Li / Zhuqiang Zhang / Xinzheng ...Authors: Chao-Pei Liu / Zhenyu Yu / Jun Xiong / Jie Hu / Aoqun Song / Dongbo Ding / Cong Yu / Na Yang / Mingzhu Wang / Juan Yu / Peini Hou / Kangning Zeng / Zhenyu Li / Zhuqiang Zhang / Xinzheng Zhang / Wei Li / Zhiguo Zhang / Bing Zhu / Guohong Li / Rui-Ming Xu /   Abstract: Chromatin inheritance entails de novo nucleosome assembly after DNA replication by chromatin assembly factor-1 (CAF-1). Yet direct knowledge about CAF-1's histone binding mode and nucleosome assembly ...Chromatin inheritance entails de novo nucleosome assembly after DNA replication by chromatin assembly factor-1 (CAF-1). Yet direct knowledge about CAF-1's histone binding mode and nucleosome assembly process is lacking. In this work, we report the crystal structure of human CAF-1 in the absence of histones and the cryo-electron microscopy structure of CAF-1 in complex with histones H3 and H4. One histone H3-H4 heterodimer is bound by one CAF-1 complex mainly through the p60 subunit and the acidic domain of the p150 subunit. We also observed a dimeric CAF-1-H3-H4 supercomplex in which two H3-H4 heterodimers are poised for tetramer assembly and discovered that CAF-1 facilitates right-handed DNA wrapping of H3-H4 tetramers. These findings signify the involvement of DNA in H3-H4 tetramer formation and suggest a right-handed nucleosome precursor in chromatin replication. | |||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33625.map.gz emd_33625.map.gz | 32 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33625-v30.xml emd-33625-v30.xml emd-33625.xml emd-33625.xml | 26.8 KB 26.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33625_fsc.xml emd_33625_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_33625.png emd_33625.png | 111.4 KB | ||
| Masks |  emd_33625_msk_1.map emd_33625_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_33625_additional_1.map.gz emd_33625_additional_1.map.gz emd_33625_half_map_1.map.gz emd_33625_half_map_1.map.gz emd_33625_half_map_2.map.gz emd_33625_half_map_2.map.gz | 59.6 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33625 http://ftp.pdbj.org/pub/emdb/structures/EMD-33625 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33625 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33625 | HTTPS FTP |
-Validation report
| Summary document |  emd_33625_validation.pdf.gz emd_33625_validation.pdf.gz | 878.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33625_full_validation.pdf.gz emd_33625_full_validation.pdf.gz | 877.9 KB | Display | |
| Data in XML |  emd_33625_validation.xml.gz emd_33625_validation.xml.gz | 15.1 KB | Display | |
| Data in CIF |  emd_33625_validation.cif.gz emd_33625_validation.cif.gz | 20.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33625 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33625 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33625 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33625 | HTTPS FTP |
-Related structure data
| Related structure data |  7y5uMC  7y5kC  7y5lC  7y5oC  7y5vC  7y5wC  7y60C  7y61C  8iqfC  8iqgC  8j6sC  8j6tC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33625.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33625.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
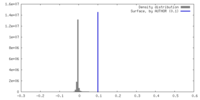
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_33625_msk_1.map emd_33625_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
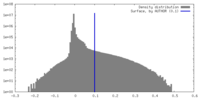
| Density Histograms |
-Additional map: sharped map
| File | emd_33625_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharped map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half B map
| File | emd_33625_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_B map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half A map
| File | emd_33625_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_A map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Monomeric CAF1L-H3-H4 complex
| Entire | Name: Monomeric CAF1L-H3-H4 complex |
|---|---|
| Components |
|
-Supramolecule #1: Monomeric CAF1L-H3-H4 complex
| Supramolecule | Name: Monomeric CAF1L-H3-H4 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 168 KDa |
-Supramolecule #2: CAF1L/RBBP4/Chromatin assembly factor 1 subunit B
| Supramolecule | Name: CAF1L/RBBP4/Chromatin assembly factor 1 subunit B / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: H3-H4
| Supramolecule | Name: H3-H4 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4-#5 |
|---|
-Macromolecule #1: Chromatin assembly factor 1 subunit A
| Macromolecule | Name: Chromatin assembly factor 1 subunit A / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.228848 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KAEITRFFQK PKTPQAPKTL AGSCGKFAPF EIKEHMVLAP RRRTAFHPDL CSQLDQLLQQ QSGEFSFLKD LKGRQPLRSG PTHVSTRNA DIFNSDVVIV ERGKGDGVPE RRKFGRMKLL QFCENHRPAY WGTWNKKTAL IRARDPWAQD TKLLDYEVDS D EEWEEEEP ...String: KAEITRFFQK PKTPQAPKTL AGSCGKFAPF EIKEHMVLAP RRRTAFHPDL CSQLDQLLQQ QSGEFSFLKD LKGRQPLRSG PTHVSTRNA DIFNSDVVIV ERGKGDGVPE RRKFGRMKLL QFCENHRPAY WGTWNKKTAL IRARDPWAQD TKLLDYEVDS D EEWEEEEP GESLSHSEGD DDDDMGEDED EDDGFFVPHG YLSEDEGVTE ECADPENHKV RQKLKAKEWD EFLAKGKRFR VL QPVKIGC VWAADRDCAG DDLKVLQQFA ACFLETLPAQ EEQTPKASKR ERRDEQILAQ LLPLLHGNVN GSKVIIREFQ EHC RRGLLS NHTGSPRSPS TTYLHTPTPS EDAAIPSKSR LKRLISENSV YEKRPDFRMC WYVHPQVLQS FQQEHLPVPC QWSY VTSVP SAPKEDS UniProtKB: Chromatin assembly factor 1 subunit A |
-Macromolecule #2: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.437167 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEACEA YLVGLFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #3: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #4: Histone-binding protein RBBP4
| Macromolecule | Name: Histone-binding protein RBBP4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.709527 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MADKEAAFDD AVEERVINEE YKIWKKNTPF LYDLVMTHAL EWPSLTAQWL PDVTRPEGKD FSIHRLVLGT HTSDEQNHLV IASVQLPND DAQFDASHYD SEKGEFGGFG SVSGKIEIEI KINHEGEVNR ARYMPQNPCI IATKTPSSDV LVFDYTKHPS K PDPSGECN ...String: MADKEAAFDD AVEERVINEE YKIWKKNTPF LYDLVMTHAL EWPSLTAQWL PDVTRPEGKD FSIHRLVLGT HTSDEQNHLV IASVQLPND DAQFDASHYD SEKGEFGGFG SVSGKIEIEI KINHEGEVNR ARYMPQNPCI IATKTPSSDV LVFDYTKHPS K PDPSGECN PDLRLRGHQK EGYGLSWNPN LSGHLLSASD DHTICLWDIS AVPKEGKVVD AKTIFTGHTA VVEDVSWHLL HE SLFGSVA DDQKLMIWDT RSNNTSKPSH SVDAHTAEVN CLSFNPYSEF ILATGSADKT VALWDLRNLK LKLHSFESHK DEI FQVQWS PHNETILASS GTDRRLNVWD LSKIGEEQSP EDAEDGPPEL LFIHGGHTAK ISDFSWNPNE PWVICSVSED NIMQ VWQMA ENIYNDEDPE GSVDPEGQGS UniProtKB: Histone-binding protein RBBP4 |
-Macromolecule #5: Chromatin assembly factor 1 subunit B
| Macromolecule | Name: Chromatin assembly factor 1 subunit B / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.714941 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKVITCEIAW HNKEPVYSLD FQHGTAGRIH RLASAGVDTN VRIWKVEKGP DGKAIVEFLS NLARHTKAVN VVRFSPTGEI LASGGDDAV ILLWKVNDNK EPEQIAFQDE DEAQLNKENW TVVKTLRGHL EDVYDICWAT DGNLMASASV DNTAIIWDVS K GQKISIFN ...String: MKVITCEIAW HNKEPVYSLD FQHGTAGRIH RLASAGVDTN VRIWKVEKGP DGKAIVEFLS NLARHTKAVN VVRFSPTGEI LASGGDDAV ILLWKVNDNK EPEQIAFQDE DEAQLNKENW TVVKTLRGHL EDVYDICWAT DGNLMASASV DNTAIIWDVS K GQKISIFN EHKSYVQGVT WDPLGQYVAT LSCDRVLRVY SIQKKRVAFN VSKMLSGIGA EGEARSYRMF HDDSMKSFFR RL SFTPDGS LLLTPAGCVE SGENVMNTTY VFSRKNLKRP IAHLPCPGKA TLAVRCCPVY FELRPVVETG VELMSLPYRL VFA VASEDS VLLYDTQQSF PFGYVSNIHY HTLSDISWSS DGAFLAISST DGYCSFVTFE KDELGIPLKE KPVLNMRTPD TAKK TKSQT HRGSSPGPRP VEGT UniProtKB: Chromatin assembly factor 1 subunit B |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20 mM Hepes, pH 7.5, 50 mM NaCl and 1 mM DTT |
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK I |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)