[English] 日本語
 Yorodumi
Yorodumi- EMDB-33521: Cryo-EM structure of Fft3-nucleosome complex with Fft3 bound to S... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Fft3-nucleosome complex with Fft3 bound to SHL+3 position of the nucleosome | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA binding / remodeler / nucleosome / Fft3-nucleosome complex / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationattachment of telomeric heterochromatin to nuclear envelope / ATP-dependent H3-H4 histone complex chaperone activity / HDMs demethylate histones / PKMTs methylate histone lysines / Interleukin-7 signaling / Chromatin modifying enzymes / Condensation of Prophase Chromosomes / SUMOylation of chromatin organization proteins / Metalloprotease DUBs / E3 ubiquitin ligases ubiquitinate target proteins ...attachment of telomeric heterochromatin to nuclear envelope / ATP-dependent H3-H4 histone complex chaperone activity / HDMs demethylate histones / PKMTs methylate histone lysines / Interleukin-7 signaling / Chromatin modifying enzymes / Condensation of Prophase Chromosomes / SUMOylation of chromatin organization proteins / Metalloprotease DUBs / E3 ubiquitin ligases ubiquitinate target proteins / Factors involved in megakaryocyte development and platelet production / RCAF complex / RMTs methylate histone arginines / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / polytene chromosome band / SIRT1 negatively regulates rRNA expression / NoRC negatively regulates rRNA expression / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / Formation of the beta-catenin:TCF transactivating complex / PRC2 methylates histones and DNA / HDACs deacetylate histones / Ub-specific processing proteases / RNA Polymerase I Promoter Escape / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / Regulation of endogenous retroelements by KRAB-ZFP proteins / larval somatic muscle development / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / Senescence-Associated Secretory Phenotype (SASP) / Transcriptional regulation by small RNAs / Estrogen-dependent gene expression / HATs acetylate histones / UCH proteinases / Assembly of the ORC complex at the origin of replication / Oxidative Stress Induced Senescence / polytene chromosome / histone chaperone activity / DNA double-strand break processing / nucleosome array spacer activity / nucleosomal DNA binding / nuclear chromosome / transcription elongation-coupled chromatin remodeling / DNA repair-dependent chromatin remodeling / replication fork processing / heterochromatin / helicase activity / structural constituent of chromatin / nucleosome / heterochromatin formation / nucleosome assembly / chromosome / chromatin organization / DNA helicase / damaged DNA binding / chromatin remodeling / protein heterodimerization activity / chromatin binding / chromatin / protein-containing complex binding / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / DNA binding / ATP binding / nucleus Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Nan Z / Tao J / Yangao H | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of Fft3-nucleosome complex with Fft3 bound to SHL+3 position of the nucleosome (Class II Fft3-nucleosome complex) Authors: Nan Z / Tao J / Yangao H | |||||||||
| History |
|
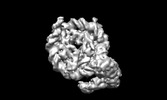
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33521.map.gz emd_33521.map.gz | 40 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33521-v30.xml emd-33521-v30.xml emd-33521.xml emd-33521.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33521.png emd_33521.png | 196.3 KB | ||
| Filedesc metadata |  emd-33521.cif.gz emd-33521.cif.gz | 7.1 KB | ||
| Others |  emd_33521_half_map_1.map.gz emd_33521_half_map_1.map.gz emd_33521_half_map_2.map.gz emd_33521_half_map_2.map.gz | 33 MB 33.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33521 http://ftp.pdbj.org/pub/emdb/structures/EMD-33521 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33521 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33521 | HTTPS FTP |
-Validation report
| Summary document |  emd_33521_validation.pdf.gz emd_33521_validation.pdf.gz | 882.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33521_full_validation.pdf.gz emd_33521_full_validation.pdf.gz | 882.2 KB | Display | |
| Data in XML |  emd_33521_validation.xml.gz emd_33521_validation.xml.gz | 11.3 KB | Display | |
| Data in CIF |  emd_33521_validation.cif.gz emd_33521_validation.cif.gz | 13.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33521 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33521 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33521 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33521 | HTTPS FTP |
-Related structure data
| Related structure data |  7xygMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33521.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33521.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
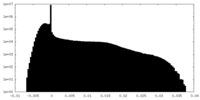
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33521_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
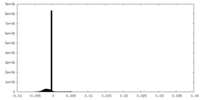
| Density Histograms |
-Half map: #1
| File | emd_33521_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
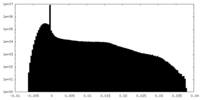
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of Fft3-nucleosome complex with Fft3 bound to SHL+3 of th...
| Entire | Name: Complex of Fft3-nucleosome complex with Fft3 bound to SHL+3 of the nucleosome (Class II Fft3-nucleosome complex) |
|---|---|
| Components |
|
-Supramolecule #1: Complex of Fft3-nucleosome complex with Fft3 bound to SHL+3 of th...
| Supramolecule | Name: Complex of Fft3-nucleosome complex with Fft3 bound to SHL+3 of the nucleosome (Class II Fft3-nucleosome complex) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #6-#7, #1-#5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Histone H2A
| Macromolecule | Name: Histone H2A / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.257529 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKGGKVK GKAKSRSNRA GLQFPVGRIH RLLRKGNYAE RVGAGAPVYL AAVMEYLAAE VLELAGNAAR DNKKTRIIPR HLQLAIRND EELNKLLSGV TIAQGGVLPN IQAVLLPKKT EKKA UniProtKB: Histone H2A |
-Macromolecule #2: Histone H2B
| Macromolecule | Name: Histone H2B / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.727064 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPPKTSGKAA KKAGKAQKNI TKTDKKKKRK RKESYAIYIY KVLKQVHPDT GISSKAMSIM NSFVNDIFER IAAEASRLAH YNKRSTITS REIQTAVRLL LPGELAKHAV SEGTKAVTKY TSSK UniProtKB: Histone H2B |
-Macromolecule #5: ATP-dependent helicase fft3
| Macromolecule | Name: ATP-dependent helicase fft3 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 104.629953 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDGKRKIEHT ADGTHYDATS NVKRKPIFPP FIADSLSEAT EKANVMGGGM NSRLQILSEM SKRVQATAPI SSLEHFKQLS DISPSFTSS ANSINQPYNY SGSLENLVPT PSAGTPSQFM DAQNPYGAVY NALSQFSETE PKMPSYMDDE EASDSLPLSL S SQSLSSQV ...String: MDGKRKIEHT ADGTHYDATS NVKRKPIFPP FIADSLSEAT EKANVMGGGM NSRLQILSEM SKRVQATAPI SSLEHFKQLS DISPSFTSS ANSINQPYNY SGSLENLVPT PSAGTPSQFM DAQNPYGAVY NALSQFSETE PKMPSYMDDE EASDSLPLSL S SQSLSSQV TNQKPAPHRL TMRERYAANN LTNGLQFTLP LSSRKTYEPE ADDDSNDDMY SDDDSNADRW ASRIDTAALK EE VLKYMNR CSTQDLADMT GCTLAEAEFM VAKRPFPDLE SALVVKQPRP VIPKGRRGRR EKTPLGPRLV GICMEIMRGY FVV DALIRQ CEQLGGKIQR GIEAWGLSNT ATSDEGETSL VNFDQMKSFG TPANSSFITT PPASFSPDIK LQDYQIIGIN WLYL LYELK LAGILADEMG LGKTCQTIAF FSLLMDKNIN GPHLVIAPAS TMENWLREFA KFCPKLKIEL YYGSQVEREE IRERI NSNK DSYNVMLTTY RLAATSKADR LFLRNQKFNV CVYDEGHYLK NRASERYRHL MSIPADFRVL LTGTPLQNNL KELISL LAF ILPHVFDYGL KSLDVIFTMK KSPESDFERA LLSEQRVSRA KMMMAPFVLR RKKSQVLDAL PKKTRIIEFC EFSEEER RR YDDFASKQSV NSLLDENVMK TNLDTNANLA KKKSTAGFVL VQLRKLADHP MLFRIHYKDD ILRQMAKAIM NEPQYKKA N ELYIFEDMQY MSDIELHNLC CKFPSINSFQ LKDEPWMDAT KVRKLKKLLT NAVENGDRVV LFSQFTQVLD ILQLVMKSL NLKFLRFDGS TQVDFRQDLI DQFYADESIN VFLLSTKAGG FGINLACANM VILYDVSFNP FDDLQAEDRA HRVGQKKEVT VYKFVVKDT IEEHIQRLAN AKIALDATLS GNAETVEAED DDD UniProtKB: ATP-dependent helicase fft3 |
-Macromolecule #6: Histone H3
| Macromolecule | Name: Histone H3 / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.289904 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ARTKQTARKS TGGKAPRKQL ATKAARKSAP ATGGVKKPHR YRPGTVALRE IRRYQKSTEL LIRKLPFQRL VREIAQDFKT DLRFQSSAV MALQEASEAY LVGLFEDTNL CAIHAKRVTI MPKDIQLARR IRGERA UniProtKB: Histone H3 |
-Macromolecule #7: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 7 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.408452 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #3: DNA (167-MER)
| Macromolecule | Name: DNA (167-MER) / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 51.783977 KDa |
| Sequence | String: (DA)(DT)(DC)(DT)(DA)(DC)(DA)(DT)(DG)(DC) (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA) (DC)(DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG) (DA)(DG)(DA)(DC)(DT)(DA) ...String: (DA)(DT)(DC)(DT)(DA)(DC)(DA)(DT)(DG)(DC) (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA) (DC)(DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG) (DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG) (DG)(DA)(DG)(DT)(DA)(DA)(DT)(DC)(DC)(DC) (DC)(DT) (DT)(DG)(DG)(DC)(DG)(DG)(DT) (DT)(DA)(DA)(DA)(DA)(DC)(DG)(DC)(DG)(DG) (DG)(DG)(DG) (DA)(DC)(DA)(DG)(DC)(DG) (DC)(DG)(DT)(DA)(DC)(DG)(DT)(DG)(DC)(DG) (DT)(DT)(DT)(DA) (DA)(DG)(DC)(DG)(DG) (DT)(DG)(DC)(DT)(DA)(DG)(DA)(DG)(DC)(DT) (DG)(DT)(DC)(DT)(DA) (DC)(DG)(DA)(DC) (DC)(DA)(DA)(DT)(DT)(DG)(DA)(DG)(DC)(DG) (DG)(DC)(DC)(DT)(DC)(DG) (DG)(DC)(DA) (DC)(DC)(DG)(DG)(DG)(DA)(DT)(DT)(DC)(DT) (DC)(DC)(DA)(DG)(DG)(DG)(DC) (DG)(DG) (DC)(DC)(DG)(DA)(DT) |
-Macromolecule #4: DNA (167-MER)
| Macromolecule | Name: DNA (167-MER) / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 51.325676 KDa |
| Sequence | String: (DA)(DT)(DC)(DG)(DG)(DC)(DC)(DG)(DC)(DC) (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG) (DA)(DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA) (DT)(DT)(DG)(DG)(DT)(DC) ...String: (DA)(DT)(DC)(DG)(DG)(DC)(DC)(DG)(DC)(DC) (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG) (DA)(DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT) (DA)(DG)(DA)(DC)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG) (DC)(DA)(DC)(DC)(DG)(DC)(DT) (DT)(DA)(DA)(DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC) (DG)(DC)(DG)(DC)(DT)(DG) (DT)(DC)(DC)(DC)(DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA) (DA)(DC)(DC)(DG)(DC) (DC)(DA)(DA)(DG)(DG)(DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC) (DC)(DT)(DA)(DG) (DT)(DC)(DT)(DC)(DC)(DA)(DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC) (DA)(DG)(DA) (DT)(DA)(DT)(DA)(DT)(DA)(DC)(DA)(DT)(DC) (DC)(DT)(DG)(DT)(DG)(DC)(DA) (DT)(DG) (DT)(DA)(DG)(DA)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.5 µm |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.2 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 49996 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)