[English] 日本語
 Yorodumi
Yorodumi- EMDB-33192: Structure of the SecA/SecYE/proOmpA(4Y)-sfGFP complex with ADP.BeF3-. -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the SecA/SecYE/proOmpA(4Y)-sfGFP complex with ADP.BeF3-. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SecA ATPase / membrane protein / TRANSLOCATE / TRANSLOCASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein-exporting ATPase activity / cell envelope Sec protein transport complex / protein-secreting ATPase / protein transport by the Sec complex / intracellular protein transmembrane transport / protein import / protein secretion / protein transmembrane transporter activity / protein targeting / membrane raft ...protein-exporting ATPase activity / cell envelope Sec protein transport complex / protein-secreting ATPase / protein transport by the Sec complex / intracellular protein transmembrane transport / protein import / protein secretion / protein transmembrane transporter activity / protein targeting / membrane raft / ATP binding / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Geobacillus thermodenitrificans NG80-2 (bacteria) / Geobacillus thermodenitrificans NG80-2 (bacteria) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.35 Å | |||||||||
 Authors Authors | Dong L / Li L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Structural basis of SecA-mediated protein translocation. Authors: Linlin Dong / Song Yang / Jingxia Chen / Xiaofei Wu / Dongjie Sun / Chen Song / Long Li /  Abstract: Secretory proteins are cotranslationally or posttranslationally translocated across lipid membranes via a protein-conducting channel named SecY in prokaryotes and Sec61 in eukaryotes. The vast ...Secretory proteins are cotranslationally or posttranslationally translocated across lipid membranes via a protein-conducting channel named SecY in prokaryotes and Sec61 in eukaryotes. The vast majority of secretory proteins in bacteria are driven through the channel posttranslationally by SecA, a highly conserved ATPase. How a polypeptide chain is moved by SecA through the SecY channel is poorly understood. Here, we report electron cryomicroscopy structures of the active SecA-SecY translocon with a polypeptide substrate. The substrate is captured in different translocation states when clamped by SecA with different nucleotides. Upon binding of an ATP analog, SecA undergoes global conformational changes to push the polypeptide substrate toward the channel in a way similar to how the RecA-like helicases translocate their nucleic acid substrates. The movements of the polypeptide substrates in the SecA-SecY translocon share a similar structural basis to those in the ribosome-SecY complex during cotranslational translocation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33192.map.gz emd_33192.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33192-v30.xml emd-33192-v30.xml emd-33192.xml emd-33192.xml | 24.2 KB 24.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33192.png emd_33192.png | 103 KB | ||
| Filedesc metadata |  emd-33192.cif.gz emd-33192.cif.gz | 7.2 KB | ||
| Others |  emd_33192_half_map_1.map.gz emd_33192_half_map_1.map.gz emd_33192_half_map_2.map.gz emd_33192_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33192 http://ftp.pdbj.org/pub/emdb/structures/EMD-33192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33192 | HTTPS FTP |
-Related structure data
| Related structure data |  7xhaMC  7xhbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33192.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33192.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.055 Å | ||||||||||||||||||||||||||||||||||||
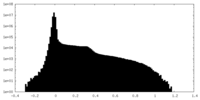
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33192_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
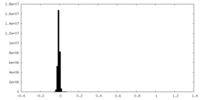
| Density Histograms |
-Half map: #1
| File | emd_33192_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
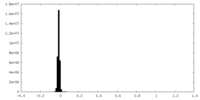
| Density Histograms |
- Sample components
Sample components
+Entire : Complex of the SecA/SecYE/proOmpA(4Y)-sfGFP with ADP.BeF3-
+Supramolecule #1: Complex of the SecA/SecYE/proOmpA(4Y)-sfGFP with ADP.BeF3-
+Supramolecule #2: SecA
+Supramolecule #3: SecY
+Supramolecule #4: SecE
+Supramolecule #5: sfGFP
+Macromolecule #1: Protein translocase subunit SecA
+Macromolecule #2: Protein translocase subunit SecY
+Macromolecule #3: Protein translocase subunit SecE
+Macromolecule #4: Translocating polypeptide
+Macromolecule #5: MAGNESIUM ION
+Macromolecule #6: BERYLLIUM TRIFLUORIDE ION
+Macromolecule #7: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 57.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)