[English] 日本語
 Yorodumi
Yorodumi- EMDB-33022: Cryo-EM structure of the human TRPC5 ion channel in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human TRPC5 ion channel in complex with G alpha i3 subunits, class1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transient receptor potential / METAL TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of membrane hyperpolarization / phosphatidylserine exposure on apoptotic cell surface / Role of second messengers in netrin-1 signaling / negative regulation of dendrite morphogenesis / store-operated calcium channel activity / inositol 1,4,5 trisphosphate binding / negative regulation of adenylate cyclase activity / cation channel complex / actinin binding / GTP metabolic process ...regulation of membrane hyperpolarization / phosphatidylserine exposure on apoptotic cell surface / Role of second messengers in netrin-1 signaling / negative regulation of dendrite morphogenesis / store-operated calcium channel activity / inositol 1,4,5 trisphosphate binding / negative regulation of adenylate cyclase activity / cation channel complex / actinin binding / GTP metabolic process / clathrin binding / TRP channels / positive regulation of macroautophagy / regulation of cytosolic calcium ion concentration / positive regulation of axon extension / Adenylate cyclase inhibitory pathway / positive regulation of neuron differentiation / calcium channel complex / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / calcium ion transmembrane transport / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / calcium channel activity / G-protein beta/gamma-subunit complex binding / neuron differentiation / ADP signalling through P2Y purinoceptor 12 / GDP binding / calcium ion transport / G alpha (z) signalling events / ADORA2B mediated anti-inflammatory cytokines production / nervous system development / GPER1 signaling / heterotrimeric G-protein complex / presynapse / actin binding / growth cone / ATPase binding / positive regulation of cytosolic calcium ion concentration / neuron apoptotic process / midbody / G alpha (i) signalling events / G alpha (s) signalling events / Extra-nuclear estrogen signaling / ciliary basal body / cell division / lysosomal membrane / neuronal cell body / GTPase activity / positive regulation of cell population proliferation / dendrite / centrosome / GTP binding / nucleolus / Golgi apparatus / extracellular exosome / nucleoplasm / metal ion binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.93 Å | |||||||||
 Authors Authors | Won J / Jeong H / Lee HH | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Molecular architecture of the Gα-bound TRPC5 ion channel. Authors: Jongdae Won / Jinsung Kim / Hyeongseop Jeong / Jinhyeong Kim / Shasha Feng / Byeongseok Jeong / Misun Kwak / Juyeon Ko / Wonpil Im / Insuk So / Hyung Ho Lee /   Abstract: G-protein coupled receptors (GPCRs) and ion channels serve as key molecular switches through which extracellular stimuli are transformed into intracellular effects, and it has long been postulated ...G-protein coupled receptors (GPCRs) and ion channels serve as key molecular switches through which extracellular stimuli are transformed into intracellular effects, and it has long been postulated that ion channels are direct effector molecules of the alpha subunit of G-proteins (Gα). However, no complete structural evidence supporting the direct interaction between Gα and ion channels is available. Here, we present the cryo-electron microscopy structures of the human transient receptor potential canonical 5 (TRPC5)-Gα complexes with a 4:4 stoichiometry in lipid nanodiscs. Remarkably, Gα binds to the ankyrin repeat edge of TRPC5 ~ 50 Å away from the cell membrane. Electrophysiological analysis shows that Gα increases the sensitivity of TRPC5 to phosphatidylinositol 4,5-bisphosphate (PIP), thereby rendering TRPC5 more easily opened in the cell membrane, where the concentration of PIP is physiologically regulated. Our results demonstrate that ion channels are one of the direct effector molecules of Gα proteins triggered by GPCR activation-providing a structural framework for unraveling the crosstalk between two major classes of transmembrane proteins: GPCRs and ion channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33022.map.gz emd_33022.map.gz | 64.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33022-v30.xml emd-33022-v30.xml emd-33022.xml emd-33022.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33022.png emd_33022.png | 163.7 KB | ||
| Filedesc metadata |  emd-33022.cif.gz emd-33022.cif.gz | 6.8 KB | ||
| Others |  emd_33022_additional_1.map.gz emd_33022_additional_1.map.gz emd_33022_additional_2.map.gz emd_33022_additional_2.map.gz | 117.7 MB 117.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33022 http://ftp.pdbj.org/pub/emdb/structures/EMD-33022 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33022 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33022 | HTTPS FTP |
-Related structure data
| Related structure data |  7x6iMC  7x6cC  8gvwC  8gvxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33022.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33022.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.088 Å | ||||||||||||||||||||||||||||||||||||
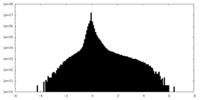
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #2
| File | emd_33022_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
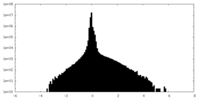
| Density Histograms |
-Additional map: #1
| File | emd_33022_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
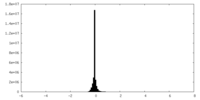
| Density Histograms |
- Sample components
Sample components
+Entire : TRP channel-G protein complex
+Supramolecule #1: TRP channel-G protein complex
+Supramolecule #2: TRP channel
+Supramolecule #3: G protein
+Macromolecule #1: Short transient receptor potential channel 5
+Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-3
+Macromolecule #3: PHOSPHATIDYLETHANOLAMINE
+Macromolecule #4: CHOLESTEROL HEMISUCCINATE
+Macromolecule #5: ZINC ION
+Macromolecule #6: CALCIUM ION
+Macromolecule #7: (2S)-2-(hexadecanoyloxy)-3-hydroxypropyl (9Z)-octadec-9-enoate
+Macromolecule #8: GUANOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.93 Å / Resolution method: OTHER Details: We combined two different maps from the same dataset (D_1300028166_em-additional-volume_P1.map.V2 and D_1300028166_em-additional-volume_P2.map.V2) to generate a composite map (D_1300028166_ ...Details: We combined two different maps from the same dataset (D_1300028166_em-additional-volume_P1.map.V2 and D_1300028166_em-additional-volume_P2.map.V2) to generate a composite map (D_1300028166_em-volume_P1.map.V2). The density of the G protein area could not be visualized clearly in the consensus map of this EM dataset. Therefore, we performed focused classification and local refinement to improve the density of the G protein area using symmetry expanded particles with C4 symmetry imposition, which required more number of particles. Finally, the number of particles used to reconstruct additional volume data 2 (D_1300028166_em-additional-volume_P2.map.V2) is 18,935 and the number of particles used to reconstruct additional volume data 1 (D_1300028166_em-additional-volume_P1.map.V2) is 205,343. Furthermore, the resolution stated above is based on map resolution estimates calculated by a validation tool in Phenix, FSC (model) = 0.5. Number images used: 18935 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)