+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GPR domain of Drosophila P5CS filament with glutamate | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glutamate-bound / filament / ALDH18A1 / Delta-1-pyrroline-5-carboxylate synthase / BIOSYNTHETIC PROTEIN / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationGlutamate and glutamine metabolism / Mitochondrial protein degradation / glutamate-5-semialdehyde dehydrogenase / glutamate-5-semialdehyde dehydrogenase activity / glutamate 5-kinase / glutamate 5-kinase activity / L-proline biosynthetic process / : / mitochondrial matrix / mitochondrion / ATP binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
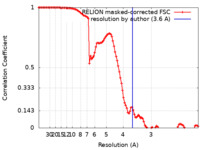
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Liu JL / Zhong J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2022 Journal: Elife / Year: 2022Title: Structural basis of dynamic P5CS filaments. Authors: Jiale Zhong / Chen-Jun Guo / Xian Zhou / Chia-Chun Chang / Boqi Yin / Tianyi Zhang / Huan-Huan Hu / Guang-Ming Lu / Ji-Long Liu /  Abstract: The bifunctional enzyme Δ-pyrroline-5-carboxylate synthase (P5CS) is vital to the synthesis of proline and ornithine, playing an essential role in human health and agriculture. Pathogenic mutations ...The bifunctional enzyme Δ-pyrroline-5-carboxylate synthase (P5CS) is vital to the synthesis of proline and ornithine, playing an essential role in human health and agriculture. Pathogenic mutations in the P5CS gene (ALDH18A1) lead to neurocutaneous syndrome and skin relaxation connective tissue disease in humans, and P5CS deficiency seriously damages the ability to resist adversity in plants. We have recently found that P5CS forms cytoophidia in vivo and filaments in vitro. However, it is difficult to appreciate the function of P5CS filamentation without precise structures. Using cryo-electron microscopy, here we solve the structures of full-length P5CS in three states at resolution from 3.1 to 4.3 Å. We observe distinct ligand-binding states and conformational changes for the GK and GPR domains, respectively. Divergent helical filaments are assembled by P5CS tetramers and stabilized by multiple interfaces. Point mutations disturbing those interfaces prevent P5CS filamentation and greatly reduce the enzymatic activity. Our findings reveal that filamentation is crucial for the coordination between the GK and GPR domains, providing a structural basis for the catalytic function of P5CS filaments. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32877.map.gz emd_32877.map.gz | 62.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32877-v30.xml emd-32877-v30.xml emd-32877.xml emd-32877.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32877_fsc.xml emd_32877_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_32877.png emd_32877.png | 87.5 KB | ||
| Masks |  emd_32877_msk_1.map emd_32877_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-32877.cif.gz emd-32877.cif.gz | 5.5 KB | ||
| Others |  emd_32877_half_map_1.map.gz emd_32877_half_map_1.map.gz emd_32877_half_map_2.map.gz emd_32877_half_map_2.map.gz | 79.1 MB 79.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32877 http://ftp.pdbj.org/pub/emdb/structures/EMD-32877 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32877 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32877 | HTTPS FTP |
-Validation report
| Summary document |  emd_32877_validation.pdf.gz emd_32877_validation.pdf.gz | 766.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32877_full_validation.pdf.gz emd_32877_full_validation.pdf.gz | 766.2 KB | Display | |
| Data in XML |  emd_32877_validation.xml.gz emd_32877_validation.xml.gz | 17.8 KB | Display | |
| Data in CIF |  emd_32877_validation.cif.gz emd_32877_validation.cif.gz | 23.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32877 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32877 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32877 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32877 | HTTPS FTP |
-Related structure data
| Related structure data |  7wxfMC  7f5tC  7f5uC  7f5vC  7f5xC  7wx3C  7wx4C  7wxgC  7wxhC  7wxiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32877.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32877.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_32877_msk_1.map emd_32877_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_32877_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_32877_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Drosophila glutamate-bound Delta-1-pyrroline-5-carboxylate synthase
| Entire | Name: Drosophila glutamate-bound Delta-1-pyrroline-5-carboxylate synthase |
|---|---|
| Components |
|
-Supramolecule #1: Drosophila glutamate-bound Delta-1-pyrroline-5-carboxylate synthase
| Supramolecule | Name: Drosophila glutamate-bound Delta-1-pyrroline-5-carboxylate synthase type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Delta-1-pyrroline-5-carboxylate synthase
| Macromolecule | Name: Delta-1-pyrroline-5-carboxylate synthase / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: glutamate 5-kinase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 84.198227 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLQNSFKLAQ SLRNGFYRNA WRAFSSHGPR QPLVSPERRL EKAHPTFTER SQLKYARRLV VKLGSAVITR EDNHGLALGR LASIVEQVA ECHLEGREVM MVTSGAVAFG KQKLAQELLM SLSMRETLNP KDSKEFDGAT LEPRAAAAVG QSGLMSLYDA M FAQYGVKI ...String: MLQNSFKLAQ SLRNGFYRNA WRAFSSHGPR QPLVSPERRL EKAHPTFTER SQLKYARRLV VKLGSAVITR EDNHGLALGR LASIVEQVA ECHLEGREVM MVTSGAVAFG KQKLAQELLM SLSMRETLNP KDSKEFDGAT LEPRAAAAVG QSGLMSLYDA M FAQYGVKI AQVLVTKPDF YNEETRNNLF CTLSELISLN IVPIINTNDA VSPPMFIRDD EPAGGARRGI PIKDNDSLSA ML AAEVQAD LLILMSDVDG IYNKPPWEDG AKLMHTYTSD DSNSIEFGKK SKVGTGGMDS KVKAATWALD RGVSVVICNG MQE KAIKTI IGGRKVGTFF TEATESANAV PVEVMAENAR TGSRQMQALT PAQRASAVNT LADLLVSREK FILDANAKDL AEAQ KSGLA KPLLSRLSLN PAKLKNLSVG LKQIAEDSHK NVGRVLRRTR LADQLELKQV TVPIGVLLVI FESRPDSLPQ VAALA MASA NGLLLKGGKE AAHSNKALME LVKEALATVG AEHAVSLVST REEISDLLSM ENHIDLIIPR GSSDLVRSIQ QQSLHI PVL GHAEGVCHVY IDRDADLEKA LRIARDAKCD YPAACNAMET LLIHEDLMSG AIFGDVCNML KREGVKIYAG PRLNQQL TF GPPAAKSLKH EYGALECCIE VVPSLDEAIN HIHTYGSSHT DVIVTENDAA ARQFLGSVDS ACVFHNASSR FADGFRFG L GAEVGISTAR IHARGPVGVE GLLTTKWILE GQDHAAADFA EGGGRTWLHE TLPLD UniProtKB: Delta-1-pyrroline-5-carboxylate synthase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)