[English] 日本語
 Yorodumi
Yorodumi- EMDB-32761: Cryo-EM structure of human glucose transporter GLUT4 bound to cyt... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human glucose transporter GLUT4 bound to cytochalasin B in detergent micelles | |||||||||
 Map data Map data | Cryo-EM structure of human glucose transporter GLUT4 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glucose transporter / GLUT4 / diabetes / cytochalasin B / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationD-glucose uniporter activity / regulation of synaptic vesicle budding from presynaptic endocytic zone membrane / positive regulation of brain-derived neurotrophic factor receptor signaling pathway / white fat cell proliferation / amylopectin biosynthetic process / dehydroascorbic acid transport / glucose import in response to insulin stimulus / Cellular hexose transport / D-glucose transmembrane transporter activity / insulin-responsive compartment ...D-glucose uniporter activity / regulation of synaptic vesicle budding from presynaptic endocytic zone membrane / positive regulation of brain-derived neurotrophic factor receptor signaling pathway / white fat cell proliferation / amylopectin biosynthetic process / dehydroascorbic acid transport / glucose import in response to insulin stimulus / Cellular hexose transport / D-glucose transmembrane transporter activity / insulin-responsive compartment / D-glucose transmembrane transport / trans-Golgi network transport vesicle / short-term memory / cellular response to osmotic stress / vesicle membrane / clathrin-coated vesicle / D-glucose import / long-term memory / brown fat cell differentiation / transport across blood-brain barrier / clathrin-coated pit / multivesicular body / T-tubule / endomembrane system / cytoplasmic vesicle membrane / sarcoplasmic reticulum / Translocation of SLC2A4 (GLUT4) to the plasma membrane / trans-Golgi network / sarcolemma / Transcriptional regulation of white adipocyte differentiation / cellular response to insulin stimulus / cellular response to tumor necrosis factor / glucose homeostasis / presynapse / response to ethanol / cellular response to hypoxia / carbohydrate metabolic process / learning or memory / membrane raft / external side of plasma membrane / perinuclear region of cytoplasm / extracellular exosome / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.31 Å | |||||||||
 Authors Authors | Yuan Y / Kong F | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Cryo-EM structure of human glucose transporter GLUT4. Authors: Yafei Yuan / Fang Kong / Hanwen Xu / Angqi Zhu / Nieng Yan / Chuangye Yan /   Abstract: GLUT4 is the primary glucose transporter in adipose and skeletal muscle tissues. Its cellular trafficking is regulated by insulin signaling. Failed or reduced plasma membrane localization of GLUT4 is ...GLUT4 is the primary glucose transporter in adipose and skeletal muscle tissues. Its cellular trafficking is regulated by insulin signaling. Failed or reduced plasma membrane localization of GLUT4 is associated with diabetes. Here, we report the cryo-EM structures of human GLUT4 bound to a small molecule inhibitor cytochalasin B (CCB) at resolutions of 3.3 Å in both detergent micelles and lipid nanodiscs. CCB-bound GLUT4 exhibits an inward-open conformation. Despite the nearly identical conformation of the transmembrane domain to GLUT1, the cryo-EM structure reveals an extracellular glycosylation site and an intracellular helix that is invisible in the crystal structure of GLUT1. The structural study presented here lays the foundation for further mechanistic investigation of the modulation of GLUT4 trafficking. Our methods for cryo-EM analysis of GLUT4 will also facilitate structural determination of many other small size solute carriers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32761.map.gz emd_32761.map.gz | 25.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32761-v30.xml emd-32761-v30.xml emd-32761.xml emd-32761.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32761.png emd_32761.png | 143.4 KB | ||
| Filedesc metadata |  emd-32761.cif.gz emd-32761.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32761 http://ftp.pdbj.org/pub/emdb/structures/EMD-32761 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32761 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32761 | HTTPS FTP |
-Validation report
| Summary document |  emd_32761_validation.pdf.gz emd_32761_validation.pdf.gz | 481.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32761_full_validation.pdf.gz emd_32761_full_validation.pdf.gz | 481.3 KB | Display | |
| Data in XML |  emd_32761_validation.xml.gz emd_32761_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_32761_validation.cif.gz emd_32761_validation.cif.gz | 6.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32761 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32761 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32761 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32761 | HTTPS FTP |
-Related structure data
| Related structure data |  7wsnMC  7wsmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32761.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32761.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of human glucose transporter GLUT4 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0825 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : GLUT4
| Entire | Name: GLUT4 |
|---|---|
| Components |
|
-Supramolecule #1: GLUT4
| Supramolecule | Name: GLUT4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56 kDa/nm |
-Macromolecule #1: Solute carrier family 2, facilitated glucose transporter member 4
| Macromolecule | Name: Solute carrier family 2, facilitated glucose transporter member 4 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.108199 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKG TMPSGFQQIG SEDGEPPQQR VTGTLVLAVF SAVLGSLQFG YNIGVINAPQ KVIEQSYNET WLGRQGPEGP SSIPPGTLT TLWALSVAIF SVGGMISSFL IGIISQWLGR KRAMLVNNVL AVLGGSLMGL ANAAASYEML ILGRFLIGAY S GLTSGLVP ...String: MDYKDDDDKG TMPSGFQQIG SEDGEPPQQR VTGTLVLAVF SAVLGSLQFG YNIGVINAPQ KVIEQSYNET WLGRQGPEGP SSIPPGTLT TLWALSVAIF SVGGMISSFL IGIISQWLGR KRAMLVNNVL AVLGGSLMGL ANAAASYEML ILGRFLIGAY S GLTSGLVP MYVGEIAPTH LRGALGTLNQ LAIVIGILIA QVLGLESLLG TASLWPLLLG LTVLPALLQL VLLPFCPESP RY LYIIQNL EGPARKSLKR LTGWADVSGV LAELKDEKRK LERERPLSLL QLLGSRTHRQ PLIIAVVLQL SQQLSGINAV FYY STSIFE TAGVGQPAYA TIGAGVVNTV FTLVSVLLVE RAGRRTLHLL GLAGMCGCAI LMTVALLLLE RVPAMSYVSI VAIF GFVAF FEIGPGPIPW FIVAELFSQG PRPAAMAVAG FSNWTSNFII GMGFQYVAEA MGPYVFLLFA VLLLGFFIFT FLRVP ETRG RTFDQISAAF HRTPSLLEQE VKPSTELEYL GPDEND UniProtKB: Solute carrier family 2, facilitated glucose transporter member 4 |
-Macromolecule #3: Cytochalasin B
| Macromolecule | Name: Cytochalasin B / type: ligand / ID: 3 / Number of copies: 1 / Formula: 5RH |
|---|---|
| Molecular weight | Theoretical: 479.608 Da |
| Chemical component information | 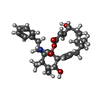 ChemComp-5RH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.8 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















