[English] 日本語
 Yorodumi
Yorodumi- EMDB-32727: Cryo-EM structure of SARS-CoV-2 Omicron spike receptor-binding do... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV-2 Omicron spike receptor-binding domain in complex with mouse ACE2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / Viral protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationMetabolism of Angiotensinogen to Angiotensins / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / maternal process involved in female pregnancy / peptidyl-dipeptidase activity ...Metabolism of Angiotensinogen to Angiotensins / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / maternal process involved in female pregnancy / peptidyl-dipeptidase activity / transporter activator activity / carboxypeptidase activity / angiotensin maturation / metallocarboxypeptidase activity / positive regulation of cardiac muscle contraction / negative regulation of smooth muscle cell proliferation / brush border membrane / negative regulation of ERK1 and ERK2 cascade / virus receptor activity / endopeptidase activity / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / apical plasma membrane / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / cell surface / extracellular space / extracellular region / metal ion binding / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
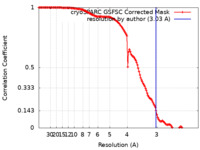
| Method | single particle reconstruction / cryo EM / Resolution: 3.03 Å | |||||||||
 Authors Authors | Han P / Xie Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2022 Journal: Cell Discov / Year: 2022Title: Broader-species receptor binding and structural bases of Omicron SARS-CoV-2 to both mouse and palm-civet ACE2s. Authors: Linjie Li / Pu Han / Baihan Huang / Yufeng Xie / Weiwei Li / Di Zhang / Pengcheng Han / Zepeng Xu / Bin Bai / Jingya Zhou / Xinrui Kang / Xiaomei Li / Anqi Zheng / Rong Zhang / Shitong Qiao ...Authors: Linjie Li / Pu Han / Baihan Huang / Yufeng Xie / Weiwei Li / Di Zhang / Pengcheng Han / Zepeng Xu / Bin Bai / Jingya Zhou / Xinrui Kang / Xiaomei Li / Anqi Zheng / Rong Zhang / Shitong Qiao / Xin Zhao / Jianxun Qi / Qihui Wang / Kefang Liu / George Fu Gao /  Abstract: The Omicron variant of SARS-CoV-2 carries multiple unusual mutations, particularly in the receptor-binding domain (RBD) of the spike (S) protein. Moreover, host-adapting mutations, such as residues ...The Omicron variant of SARS-CoV-2 carries multiple unusual mutations, particularly in the receptor-binding domain (RBD) of the spike (S) protein. Moreover, host-adapting mutations, such as residues 493, 498, and 501, were also observed in the Omicron RBD, which indicates that it is necessary to evaluate the interspecies transmission risk of the Omicron variant. Herein, we evaluated the interspecies recognition of the Omicron BA.1 and Delta RBDs by 27 ACE2 orthologs, including humans. We found that Omicron BA.1 expanded its receptor binding spectra to palm-civet, rodents, more bats (least horseshoe bat and greater horseshoe bat) and lesser hedgehog tenrec. Additionally, we determined the cryo-electron microscopy (cryo-EM) structure of the Omicron BA.1 S protein complexed with mouse ACE2 (mACE2) and the crystal structure of Omicron RBD complexed with palm-civet ACE2 (cvACE2). Several key residues for the host range have been identified. These results suggest that surveillance should be enhanced on the Omicron variant for its broader-species receptor binding to prevent spillover and expansion of reservoir hosts for a prolonged pandemic. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32727.map.gz emd_32727.map.gz | 230.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32727-v30.xml emd-32727-v30.xml emd-32727.xml emd-32727.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32727_fsc.xml emd_32727_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_32727.png emd_32727.png | 53.7 KB | ||
| Filedesc metadata |  emd-32727.cif.gz emd-32727.cif.gz | 7.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32727 http://ftp.pdbj.org/pub/emdb/structures/EMD-32727 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32727 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32727 | HTTPS FTP |
-Related structure data
| Related structure data |  7wriMC  7wrhC  7wskC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32727.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32727.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Complex of SARS-CoV-2 Omicron spike protein with mouse ACE2
| Entire | Name: Complex of SARS-CoV-2 Omicron spike protein with mouse ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of SARS-CoV-2 Omicron spike protein with mouse ACE2
| Supramolecule | Name: Complex of SARS-CoV-2 Omicron spike protein with mouse ACE2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #2: mouse ACE2
| Supramolecule | Name: mouse ACE2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: SARS-CoV-2 Omicron spike protein
| Supramolecule | Name: SARS-CoV-2 Omicron spike protein / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Processed angiotensin-converting enzyme 2
| Macromolecule | Name: Processed angiotensin-converting enzyme 2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: angiotensin-converting enzyme 2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 69.218594 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QSLTEENAKT FLNNFNQEAE DLSYQSSLAS WNYNTNITEE NAQKMSEAAA KWSAFYEEQS KTAQSFSLQE IQTPIIKRQL QALQQSGSS ALSADKNKQL NTILNTMSTI YSTGKVCNPK NPQECLLLEP GLDEIMATST DYNSRLWAWE GWRAEVGKQL R PLYEEYVV ...String: QSLTEENAKT FLNNFNQEAE DLSYQSSLAS WNYNTNITEE NAQKMSEAAA KWSAFYEEQS KTAQSFSLQE IQTPIIKRQL QALQQSGSS ALSADKNKQL NTILNTMSTI YSTGKVCNPK NPQECLLLEP GLDEIMATST DYNSRLWAWE GWRAEVGKQL R PLYEEYVV LKNEMARANN YNDYGDYWRG DYEAEGADGY NYNRNQLIED VERTFAEIKP LYEHLHAYVR RKLMDTYPSY IS PTGCLPA HLLGDMWGRF WTNLYPLTVP FAQKPNIDVT DAMMNQGWDA ERIFQEAEKF FVSVGLPHMT QGFWANSMLT EPA DGRKVV CHPTAWDLGH GDFRIKMCTK VTMDNFLTAH HEMGHIQYDM AYARQPFLLR NGANEGFHEA VGEIMSLSAA TPKH LKSIG LLPSDFQEDS ETEINFLLKQ ALTIVGTLPF TYMLEKWRWM VFRGEIPKEQ WMKKWWEMKR EIVGVVEPLP HDETY CDPA SLFHVSNDYS FIRYYTRTIY QFQFQEALCQ AAKYNGSLHK CDISNSTEAG QKLLKMLSLG NSEPWTKALE NVVGAR NMD VKPLLNYFQP LFDWLKEQNR NSFVGWNTEW SPYAD UniProtKB: Angiotensin-converting enzyme 2 |
-Macromolecule #2: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 137.201359 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QCVNLTTRTQ LPPAYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHVISGTNG TKRFDNPVLP FNDGVYFASI EKSNIIRGW IFGTTLDSKT QSLLIVNNAT NVVIKVCEFQ FCNDPFLDHK NNKSWMESEF RVYSSANNCT FEYVSQPFLM D LEGKQGNF ...String: QCVNLTTRTQ LPPAYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHVISGTNG TKRFDNPVLP FNDGVYFASI EKSNIIRGW IFGTTLDSKT QSLLIVNNAT NVVIKVCEFQ FCNDPFLDHK NNKSWMESEF RVYSSANNCT FEYVSQPFLM D LEGKQGNF KNLREFVFKN IDGYFKIYSK HTPIIVRDEP ELPQGFSALE PLVDLPIGIN ITRFQTLLAL HRSYLTPGDS SS GWTAGAA AYYVGYLQPR TFLLKYNENG TITDAVDCAL DPLSETKCTL KSFTVEKGIY QTSNFRVQPT ESIVRFPNIT NLC PFDEVF NATRFASVYA WNRKRISNCV ADYSVLYNLA PFFTFKCYGV SPTKLNDLCF TNVYADSFVI RGDEVRQIAP GQTG NIADY NYKLPDDFTG CVIAWNSNKL DSKVSGNYNY LYRLFRKSNL KPFERDISTE IYQAGNKPCN GVAGFNCYFP LRSYS FRPT YGVGHQPYRV VVLSFELLHA PATVCGPKKS TNLVKNKCVN FNFNGLKGTG VLTESNKKFL PFQQFGRDIA DTTDAV RDP QTLEILDITP CSFGGVSVIT PGTNTSNQVA VLYQGVNCTE VPVAIHADQL TPTWRVYSTG SNVFQTRAGC LIGAEYV NN SYECDIPIGA GICASYQTQT KSHRRARSVA SQSIIAYTMS LGAENSVAYS NNSIAIPTNF TISVTTEILP VSMTKTSV D CTMYICGDST ECSNLLLQYG SFCTQLKRAL TGIAVEQDKN TQEVFAQVKQ IYKTPPIKYF GGFNFSQILP DPSKPSKRS PIEDLLFNKV TLADAGFIKQ YGDCLGDIAA RDLICAQKFK GLTVLPPLLT DEMIAQYTSA LLAGTITSGW TFGAGPALQI PFPMQMAYR FNGIGVTQNV LYENQKLIAN QFNSAIGKIQ DSLSSTPSAL GKLQDVVNHN AQALNTLVKQ LSSKFGAISS V LNDIFSRL DPPEAEVQID RLITGRLQSL QTYVTQQLIR AAEIRASANL AATKMSECVL GQSKRVDFCG KGYHLMSFPQ SA PHGVVFL HVTYVPAQEK NFTTAPAICH DGKAHFPREG VFVSNGTHWF VTQRNFYEPQ IITTDNTFVS GNCDVVIGIV NNT VYDPLQ PELDSFKEEL DKYFKNHTSP DVDLGDISGI NASVVNIQKE IDRLNEVAKN LNESLIDLQE LGKYEQGSGY IPEA PRDGQ AYVRKDGEWV LLSTFLGSAW SHPQFEK UniProtKB: Spike glycoprotein |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)