+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of alphavirus, Getah virus | |||||||||
 Map data Map data | Cryo-EM structure of Getah virus, Block-based reconstruction method. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | alphavirus / Getah virus / Togaviridae / structural genomics / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationtogavirin / T=4 icosahedral viral capsid / symbiont-mediated suppression of host toll-like receptor signaling pathway / host cell cytoplasm / serine-type endopeptidase activity / fusion of virus membrane with host endosome membrane / symbiont entry into host cell / virion attachment to host cell / host cell nucleus / host cell plasma membrane ...togavirin / T=4 icosahedral viral capsid / symbiont-mediated suppression of host toll-like receptor signaling pathway / host cell cytoplasm / serine-type endopeptidase activity / fusion of virus membrane with host endosome membrane / symbiont entry into host cell / virion attachment to host cell / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / RNA binding / membrane Similarity search - Function | |||||||||
| Biological species |  Getah virus Getah virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Wang M / Sun ZZ | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Implications for the pathogenicity and antigenicity of alpha viruses revealed by a 3.5 angstrom Cryo-EM structure of Getah virus Authors: Wang M / Sun ZZ / Wang JF | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32412.map.gz emd_32412.map.gz | 267.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32412-v30.xml emd-32412-v30.xml emd-32412.xml emd-32412.xml | 10 KB 10 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32412.png emd_32412.png | 139.7 KB | ||
| Filedesc metadata |  emd-32412.cif.gz emd-32412.cif.gz | 5.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32412 http://ftp.pdbj.org/pub/emdb/structures/EMD-32412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32412 | HTTPS FTP |
-Validation report
| Summary document |  emd_32412_validation.pdf.gz emd_32412_validation.pdf.gz | 677.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32412_full_validation.pdf.gz emd_32412_full_validation.pdf.gz | 676.9 KB | Display | |
| Data in XML |  emd_32412_validation.xml.gz emd_32412_validation.xml.gz | 8.2 KB | Display | |
| Data in CIF |  emd_32412_validation.cif.gz emd_32412_validation.cif.gz | 9.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32412 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32412 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32412 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32412 | HTTPS FTP |
-Related structure data
| Related structure data |  7wc2MC  7vgaC  7wcoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32412.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32412.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of Getah virus, Block-based reconstruction method. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.36 Å | ||||||||||||||||||||||||||||||||||||
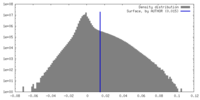
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Getah virus
| Entire | Name:  Getah virus Getah virus |
|---|---|
| Components |
|
-Supramolecule #1: Getah virus
| Supramolecule | Name: Getah virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 59300 / Sci species name: Getah virus / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Getah virus Getah virus |
-Macromolecule #1: Spike glycoprotein E1
| Macromolecule | Name: Spike glycoprotein E1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Getah virus Getah virus |
| Molecular weight | Theoretical: 47.62077 KDa |
| Sequence | String: YEHTATIPNV VGFPYKAHIE RNGFSPMTLQ LEVLGTSLEP TLNLEYITCE YKTVVPSPYI KCCGTSECRS MERPDYQCQV YTGVYPFMW GGAYCFCDTE NTQLSEAYVD RSDVCKHDHA AAYKAHTAAM KATIRISYGN LNQTTTAFVN GEHTVTVGGS R FTFGPIST ...String: YEHTATIPNV VGFPYKAHIE RNGFSPMTLQ LEVLGTSLEP TLNLEYITCE YKTVVPSPYI KCCGTSECRS MERPDYQCQV YTGVYPFMW GGAYCFCDTE NTQLSEAYVD RSDVCKHDHA AAYKAHTAAM KATIRISYGN LNQTTTAFVN GEHTVTVGGS R FTFGPIST AWTPFDNKIV VYKNDVYNQD FPPYGSGQPG RFGDIQSRTV ESKDLYANTA LKLSRPSSGT VHVPYTQTPS SF KYWIKER GTSLNDKAPF GCVIKTNPVR AENCAVGNIP VSMDIPDTAF TRVIDAPAVT NLECQVAVCT HSSDFGGIAT LTF KTDKPG KCAVHSHSNV ATIQEAAVDI KTDGKITLHF STASASPAFK VSVCSAKTTC MAACEPPKDH IVPYGASHNN QVFP DMSGT AMTWVQRVAG GLGGLTLAAV AVLILVTCVT MRR UniProtKB: Structural polyprotein |
-Macromolecule #2: Spike glycoprotein E2
| Macromolecule | Name: Spike glycoprotein E2 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Getah virus Getah virus |
| Molecular weight | Theoretical: 46.416793 KDa |
| Sequence | String: SVTEHFNVYK ATKPYLAYCA DCGDGQFCYS PVAIEKIRDE ASDGMIKIQV AAQIGINKGG THEHNKIRYI AGHDMKEANR DSLQVHTSG VCAIRGTMGH FIVAYCPPGD ELKVQFQDAE SHTQACKVQY KHAPAPVGRE KFTVRPHFGI EVPCTTYQLT T APTEEEID ...String: SVTEHFNVYK ATKPYLAYCA DCGDGQFCYS PVAIEKIRDE ASDGMIKIQV AAQIGINKGG THEHNKIRYI AGHDMKEANR DSLQVHTSG VCAIRGTMGH FIVAYCPPGD ELKVQFQDAE SHTQACKVQY KHAPAPVGRE KFTVRPHFGI EVPCTTYQLT T APTEEEID MHTPPDIPDI TLLSQQSGNV KITAGGKTIR YNCTCGSGNV GTTSSDKTIN SCKIAQCHAA VTNHDKWQYT SS FVPRADQ LSRKGKVHVP FPLTNSTCRV PVARAPGVTY GKRELTVKLH PDHPTLLTYR SLGADPRPYE EWIDRYVERT IPV TEEGIE YRWGNNPPVR LWAQLTTEGK PHGWPHEIIL YYYGLYPAAT IAAVSAAGLA VVLSLLASCY MFATARRKCL TPYA LTPGA VVPVTLGVLC CAPRAHA UniProtKB: Structural polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)